Properties of chemical elements, metals, nonmetals , metalloids
|
The number of
known elements has increased over the years. There were only 63
known elements in 1869. That was when Mendeleeff organized the first
periodic table after 20 years of research. His periodic table was organized on the basis of atomic weights. “I began to look about and write down the elements with their atomic weights and typical properties, analogous elements and like atomic weights on separate cards, and this soon convinced me that the properties of elements are in periodic dependence upon their atomic weights.” The modern periodic table is organized based on atomic numbers ( number of protons in the atomic nucleus). It has 112 elements Click here to access an interactive periodic table and there will be more. future artificial / synthetic elements There are three classifications for the elements. There are metals, nonmetals and metalloids. The pure elements are rarely found in nature. The elements are typically combined with one another to form compounds. Gold and platinum are two that are found pure in nature. Click here to see more about finding gold The vertical columns of the periodic table are called groups. The elements in a group have similar chemical properties. Examples are Group 1a where all the elements are metals and react to form positive ions. These are called the alkali metals. At the other side of the periodic table is Group 8A. These elements are nonmetals. They are typically unreactive. The Group 8A elements are He, Ne, Ar, Kr, Xe, Rn. This group has been called the rare gases or noble gases because of their relative unreactivity. click here for more about groups.
|
|
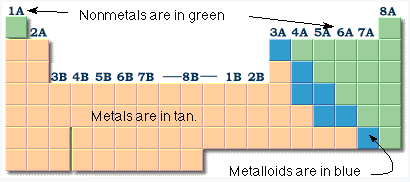
Metals are usually solids at room temperature, good conductors of heat electricity, are ductile and malleable. They make up the largest part of the periodic table. The nonmetals have the opposite properties of metals. There are seventeen (17) nonmetals. Eleven of these are gases, one is a liquid ( bromine) and five are solids at room temperature. Nonmetals are poor conductors of heat and electricity; they are neither malleable nor ductile. The seven metalloids are the smallest class of elements. The metalloids; boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At) are the elements found along the step like line between metals and non-metals of the periodic table. They have some properties like metals and some properties like nonmetals. Silicon, Si, in Group 4a is one of the most well known metalloids used to make computer chips and transistors. |
|
Metals like iron usually have high melting points. Click here for more about iron. However, mercury, Hg, has a low melting point. Click here for more about mercury, Hg. |
Metals can be hammered into thin sheets because they are malleable. Gold is one of the most malleable metals. Gold foil is used for decoration. Click here for more about gold, Au.
|
|
|
|
revised June 26, 2013 by Dr. Walt Volland