![]()
Chemical formulas: counts of atoms
Compounds are represented using formulas like H2O. These use the element symbols and subscripts. The subscripts tell the number of each type of atom. The element symbols tell what kinds of atoms are in the compound. All water molecules have this same definite composition. This is one way we can identify compounds versus mixtures. Compounds have definite composition. Natural gas is mainly methane which has the formula CH4. The molecules are small and are in the gas state at room temperature and normal sea level pressure. There are four hydrogen atoms and one carbon atom in a methane molecule. The subscripts are essential so the count of atoms of each element are correct for the compound. Benzene is a hydrocarbon compound but it is a liquid at room temperature and is a bigger molecule, C6H6. If the subscripts are changed then the formula represents a different substance. The formula for water is H2O. When the subscript on the oxygen is a '2' we get the formula for hydrogen peroxide which is H2O2. These formulas represent ONE formula unit or molecule. Increased amounts of a substance mean more of the formula units are present. 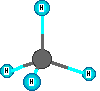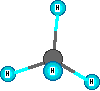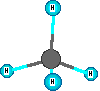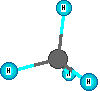
Other model examples of formulas and structures for molecules are shown here.
|