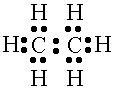|
A molecular formula
gives the types and the count of atoms for each element in a
compound. An example of a molecular formula is ethane,
C2H6.
Here the formula indicates carbon and hydrogen are combined
in ethane. The subscripts tell us that there are 2 carbon
atoms and 6 hydrogen atoms in a formula unit.
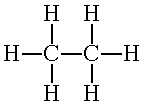
The structural
formula shows the atoms in a formula unit and the bonds
between atoms as lines. Single bonds are one line, Double
bonds are two lines. Triple bonds are three lines. The Lewis
dot structure shows the number of valence electrons and
types of bonds in the molecule.
|
Lewis
dot structure
|
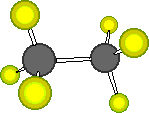
Ball
and stick model
|
|