|
Chemical Equations; reactants, products, symbols, states : reactants, Revised July 9, 2013 all rights reserved Dr. Walt Volland
|
|
Reactants and products A chemical equation uses chemical symbols for reactants and products to describe a chemical change.Reactants are the materials that are present at the beginning of a reaction. Reactants are written on the left-hand side of the equation arrow. Products are the materials formed in a chemical reaction. Products are written on the right hand side of the equation arrow. |
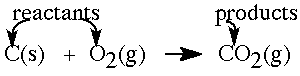 |
|
States of reactants & products The conditions for a reaction are written over the arrow. These conditions may be the temperature, addition of heat, a presence of a catalyst (a reaction promoter), etc. The physical for the reactants and products are indicated by parenthetical terms |
|
State |
Dissolved in water |
Solid |
Liquid |
Gas |
|
Symbol |
(aq) |
(s) |
(l) |
(g) |
|
The Law of Conservation of mass This means the atoms that exist today are essentially the same ones that existed thousands and millions of years ago. The atoms in use today have been recycled time after time. Everyone has been recycling with chemists since the beginning of time. Pretty cool, huh? |
|
|
|
This means the atoms that exist today are essentially the same ones that existed thousands and millions of years ago. The atoms in use today have been recycled time after time. Everyone has been recycling with chemists since the beginning of time. Pretty cool, huh? |
|
Balanced equations. |
|
The letter symbols that represent atoms and molecules in equations are treated like objects. Balancing chemical equations literally means counting the number of times atom symbols appear in the reactants and products to make sure the counts are the same on both sides. Conservation of mass is linked to "conservation " of element symbols. The law of conservation of mass is met when the count of element symbols on reactants side is equal to the count of element symbols on the product side. This rests on the additional assumption that the symbols also represent the masses of the elements. |
A balanced equation has equal counts (number) of atoms of each element in both reactants and products. Whole numbers are normally used as coefficients in balanced equations. The subscripts cannot be changed because this would alter the identity of the molecules in the equation. For example, water is H2O while hydrogen peroxide is H2O2. Similarly carbon monoxide is CO , but carbon dioxide is CO2. If the subscripts on a reactant or product are changed then the meaning of the equation will change. |
|
A balanced equation has equal counts (number) of atoms of each element in both reactants and products. Whole numbers are normally used as coefficients in balanced equations. The subscripts cannot be changed because this would alter the identity of the molecules in the equation. For example, water is H2O while hydrogen peroxide is H2O2. Similarly carbon monoxide is CO , but carbon dioxide is CO2. If the subscripts on a reactant or product are changed then the meaning of the equation will change. |
|
Example. |
|
The reaction of hydrogen, H2, and chlorine, Cl2, to make hydrogen chloride is animated below. The space filling models show how atom counts are balanced. |
