|
Molar mass and Avogadro's Number, Examples Revised October 12, 2009 Dr. Walt Volland all rights reserved 2005-2009 |
| Counting by weighing and Avogadro's number |
| The size of molecule is so small that it is physically difficult if not impossible to directly count out molecules. this problem is solved using a common trick. Atoms and molecules are counted indirectly by weighing. |
|
Here is a practical example of counting by weighing. . You need to estimate the number of nails in a box. You weigh an empty box, 213. g. The weight of the box plus nails is 1340. g. The weight of one nail is 0.450 g.I hope you aren't going to tear open the package and count the nails. We agree thatmass of nails = 1340 g - 213 g = 1227. gNumber of nails = (1227. grams nails)(1 nail/ 0.450 grams ) = 2726.6 nails = 2730 nailsYou can count the nails by weighing them. |
| Avogadro's number is an accident of nature. It is the number of particles that delivers a mole of a substance. Avogadro's number = 6.022 x 1023. |
| The reason why the value is an accident of nature is that the mole is tied to the gram mass unit. |
|
The gram is a convenient mass unit because it matches human sizes. If we were a thousand times greater in size ( like Paul Bunyan) we would find it handy to use kilogram amounts. This means the kilogram mole would be convenient. The number of particles handled in a kilogram mole is 1000 times greater. The kilo Avogadro number for the count of particles in a kilomole is 6.022 x 1026. |
|
If humans were tiny creatures (like Lilliputians) only 1/1000 our present size, milligrams would be more convenient. This means the milligram mole would be more useful. The number of particles handled in a milligram mole (millimole) would be 1/1000 times smaller. The milli Avogadro number for the count of particles in a millimole is 6.022 x 1020.
|
| What do you think would happen to Avogadro's number if the American system was used and amounts were measured in pound moles? Remember 1 pound = 454 grams. Avogadro's number would be larger by a factor of 454. A pound mole of hydrogen would weigh 1 pound which would be 454 grams. A gram mole of hydrogen weighs 1 gram and contains 6.022 x 1023 H atoms. |
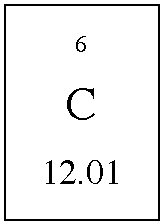
| Molar mass for elements |
| You are
able to read the periodic table and determine the average atomic mass
for an element like carbon. The average mass is 12.01 amu. This mass is
a ridiculously tiny number of grams. It is too small to handle normally.The
molar mass of carbon is defined as the mass in grams that is numerically
equal to the average atomic weight. This means
1 gram mole carbon = 12.01 grams carbon this is commonly written 1 mole carbon = 12.01 grams carbon. This is the mass of carbon that contains 6.022 x 1023 carbon atoms. Avogadro's number is 6.022 x 1023 particles. This same process gives us the molar mass of any element. 1 mole neon = 20.18 grams neon, Ne 1 mole sodium = 22.99 grams sodium, Na |
|
| Molar mass for compounds return to top of page |
|
The formulas for compounds are familiar to you. You know the formula for water is H2O. It should be reasonable that the weight of a formula unit can be calculated by adding up the weights for the atoms in the formula.The formula weight for water = weight from hydrogen + weight from oxygen
|
|
Example: The formula for methane the major component in natural gas is CH4. return to top of page The formula weight for methane = weight from hydrogen + weight from carbon
|
|
Example: The formula for ethyl chloride CH3 CH2Cl. The formula weight = weight from hydrogen + weight from carbon + weight from chlorine
|
|
Example: return to top of page |
| What is the molar mass for sulfur dioxide, SO2 (g), a gas using in bleaching and disinfection processes? |
|
| 1. Look up the atomic weight for each of the elements in the formula. |
|
|
|
| 2. Count the atoms of each element in the formula unit. . |
|
one sulfur atom ; two oxygen atoms |
| 3. The formula weight = weight from sulfur + weight from oxygen |
| 4. The formula weight = 1 sulfur atom x (32. 07 amu ) + 2 oxygen atoms x (16.00 amu) |
| 5. The formula weight SO2 = 32. 07 amu + 32.00 amu = 64.07 amu = 64 amu SO2 |
| 6. The molar mass for SO2 is 64.07 grams of SO2; 1 mol SO2 = 64 grams SO2 |
| Exercise: What is the formula weight and molar mass for alum, KAl(SO4)2• 12 H2O ? Click here for answer |