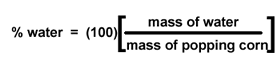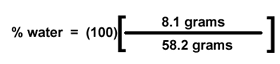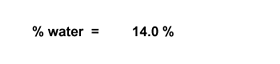Popcorn is a modern day snack but it has been around for a long time. Ears of popping corn dating back 5,600 years were found in New Mexico in 1948-1950. A 1,000 year old popped kernel of popcorn was found in a dry tomb on the east coast of Peru. More history can be reviewed using this source.
Popcorn kernels are corn
seeds consisting mostly of the carbohydrate starch; they also contain small
amounts of other substances, and a variable amount water. In the popcorn kernel,
the water molecules are found in between the granules of the starch. All substances
are enclosed in a hard shell forming what we call the kernel of popcorn. This link provides background and history about popping corn.
To understand what happens
when we pop our popcorn, a bit of background about liquids and gases
(vapors) is useful. This is studied in detail in when dealing with states of matter, but for now a short explanation
is sufficient. A liquid substance has a layer of vapor above it this is why we can smell liquids; the vapor contains
the same molecules found in the liquid, just in the form of the gas state. (Review Chapter
1 if necessary.) These gas molecules bump into and push against the walls of
the container, exerting what we call "pressure". Since these are molecules of
vapor above the liquid, this pressure is called the vapor pressure of the
liquid. Higher temperatures produce more gas molecules, this means more molecule impacts on the kernel wall. The greater
the temperature, the faster the molecules move; this means they hit the walls
more often and with more energy.
When a kernel of popcorn
is heated, the temperature of the water inside rises. Normally, at 100oC
water boils and turns to steam. However, the sealed kernel acts like a pressure
cooker and the trapped water is superheated to more than 100oC inside
the kernel. The vapor pressure of the superheated water rises until the pressure
ruptures the kernel. The pressure "pushes" against the hard shell of the kernel
until the kernel explodes or "pops". The superheated water converts totally
to steam once the kernel is forced open. The volume of the steam will be greater
than the volume of the liquid water it comes from at the same temperature. This
expanding steam "fluffs" the carbohydrates and starch into the fluffy popcorn
we enjoy eating. This is somewhat like blowing up a balloon until it breaks.
You also know that if you let air escape slowly from the balloon, it will deflate
but will not pop. Similarly, a little crack in the hard shell of the corn kernel
will keep the water inside from superheating and will let the steam leak out or escape without
expanding the kernel to its fluffy best.
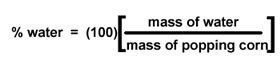
You will weigh your popcorn
before you heat it. (Chemists still say "weigh" the popcorn, even though we
mean "measure the mass of" the popcorn.) Then you will pop the corn, allowing
its water to turn to steam and expand the kernels. Once the kernel has exploded,
its water (steam) will escape to the air. When you weigh the popped corn after
it cools, its mass will be less by the number of grams of water that have escaped. |