Measurements, length, area, volume mass
Dr.Walt Volland, All rights reserved copyright 1998-2000
revised April 13, 2000
The experiment report is due 11:59 PM Saturday April 22, 2000
Measurements are a
fundamental part of science and technology. The way
measurements are made is always on a relative basis. The
medieval systems were typically established by royal decree
and often referred to the dimensions of body parts. A time
line for refinements in the definition of the meter is
at
http://www.mel.nist.gov/div821/museum/timeline.htm
Another time line is
at
http://lamar.ColoState.edu/~hillger/meter.htm
|
The metric system is relatively recent. It dates back to the period of the French Revolution. A brief description of the origins of the metric system and SI units is at this page supported by the National Institute of Standards and Technology, NIST. |
|
This experiment is intended to give you hands on practice with length, area, volume and mass units. You will use the SI units to measure length. You will measure length and width and then calculate area. You will construct a cube with each edge equal to one decimeter to see first hand how the volume of a liter is defined. You will also measure a liter of water and "feel" the heft of a kilogram. Since water has an approximate density of 1 gram /mL, the mass of the liter of water is essentially 1 kilogram. The actual volume of a 2-liter soft drink bottle will be measured. The 2-liter bottles will then be used to measure the volume of an exhaled breath. You will repeat this measurement three times and report your average value. You will also compare your average value with what your classmates report. You will explain why there are variations from person to person. You will also make suggestions for possible improvements in the procedure.
|
Supplies and Materials
|
Flexible plastic straw Ruler with metric scale showing centimeters and millimeters Measuring cup able to measure milliliters (Pyrex and other "glass" types usually have both metric and American units) Two empty, clean plastic 2-liter soft drink bottles. Large bowl, kettle, pail or other container, approximately 8-10 qt. or larger |
READ ALL THE STEPS IN THE PROCEDURE BEFORE STARTING THE EXPERIMENT
Length and area
|
Use the centimeter ruler to measure the length and width of the first page of your textbook in units of centimeters. This page has a list of the element names, symbols and average atomic masses. Record the length and width on the report sheet. Calculate the area for this page to the correct number of significant figures. Record your answer, including the proper units. Convert your area to square millimeters and record your answer. Do the number of significant figures change?
|
Volume and the decimeter
|
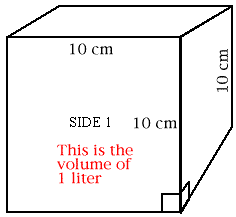
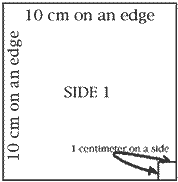
Use your centimeter ruler to draw a line 10 centimeter long. Draw another 10 cm line at a 90 degree angle to the first. Draw two more lines in the same way so you have constructed a square with 10 cm edges. Make a mark 1 cm to the left of the lower right hand corner; make a similar mark 1 cm above the lower right corner. Draw lines 1 cm long perpendicular (at 90 degrees) to the lower and the right edges of your square so you have drawn a small square 1 cm on an edge. Repeat steps 1-4 to make five more squares just like Side 1. Cut out the 6 squares and tape them together to form a cube. Place the little squares at the lower right corner in front and at the upper left rear corner of this cube. The big cube you have built is a cubic decimeter in size. It has a volume of one liter. What is the volume of the little cube whose faces you see in the lower right corner of the big cube? How many of these little cubes fit into the cubic decimeter?
|
|
|
--------------- |
------ ------------------ ------
------ ------ ------ ------ ------ ------ |
|
Remember that when length is multiplied by length the new
units are length to the power of "2". The units are squared.
|
|
Remember that when length is multiplied by length is
multiplied by length the new units are length to the power
of "3". The units are cubed.
|
Mass of one kilogram
Gather the 2-liter
soft drink bottle and your measuring cup with milliliter
calibrations. Be sure your 2-liter
bottle is clean and dry. Pick up the empty bottle and get a
sense of its weight. Use your measuring cup
to add one liter of water to the bottle. Mark the water
level with a marking pen. Lift the bottle and its contents.
Note the "heft" of the bottle and water. This mass equals a
kilogram plus the mass of the bottle. Hopefully this gives
you a feeling for a kilogram.
The volume of a breath of exhaled air
Gather the 2-liter
bottle, measuring cup, large container, and a flexible
straw. Fill the bottle to the
very top with tap water. Carefully pour water from the
bottle into the measuring cup to determine the volume of
water in milliliters that actually fits into the
bottle. You will need to transfer a number of portions of
water to the measuring cup. Be sure not to fill the cup
above its top reference mark. Keep track of the amount of
water you pour into the cup. Record the bottle's volume in
milliliters to the correct number of significant figures.
The bottle will hold more than two liters. Repeat this step two
more times. Then average the 3 values for the volume of your
bottle and record the average. Place the kettle or
other large container in the kitchen sink. Add tap water to
this container until it is about half full. Fill your 2-liter
bottle again to the very top. You can seal the bottle by
screwing the bottle cap on. Alternately you can place the
palm of your hand over the mouth of the bottle to seal the
bottle. Keep your hand tight against the mouth of the bottle
and turn the bottle upside down. The water should still be
in the bottle because you have the mouth sealed with your
hand. Keeping the bottle
upsidedown, quickly put the mouth of the bottle several
inches below the surface of the water in the large
container. You can unscrew the bottle cap to open the
bottle. You can then remove your hand from the mouth of the
bottle. The water should stay in the bottle as long as the
mouth of the bottle stays underwater. Take your flexible
straw and bend it to make a "3"
shape. The type of straw is shown here. You will need to
adjust the bend to suit your work. The coin (a penny) is
included to give an idea of the relative size of the straw.
Put the short end
inside the mouth of the bottle. You should be able to put
your mouth on the other end. Before you put your
mouth to the straw, take a deep breath and hold
it. Close your lips around
the straw and blow out as much air as you can in a steady
stream through the straw. Air bubbles will come out the end
of the straw and be trapped in the bottle as you exhale. The
air will push an equal volume of water out of the bottle,
which is why you have your large container sitting in the
sink! While the mouth of the
bottle is still underwater, place the palm of your hand over
the mouth to again seal it. Keep the bottle sealed with your
hand. Remove the bottle from the container and turn the
bottle right side up. Measure the volume of
water left in the bottle by pouring the water into your
measuring cup as you did before. Record this volume in
milliliters. Calculate the volume
of your breath by subtracting the volume of the water left
in the bottle from the total volume of the bottle. Record
the volume of breath you can exhale. Repeat until you have
three measurements of the volume of breath you can
exhale. Calculate the average
volume you can exhale and post your result to the class
conference.

Report sheet for data, results, and answers to questions.
The experiment report is due 11:59 PM Saturday January 22, 2000
|
1. Length and area |
|
Report data with appropriate number of significant figures |
|
1. |
What is the long dimension of the textbook page in centimeters?
|
cm. |
|
2. |
What is the narrow dimension of the textbook page in centimeters?
|
cm. |
|
3. |
What is the area of a page in square centimeters? set up for calculation below and the answer with units in the box to the right.
|
|
|
4. |
What is the area of a page in square millimeters? set up for calculation below and the answer in the box to the right.(This is a second order conversion, exponent is a "2" not a "1" on the units.)
|
mm2 |
|
2. Volume and the decimeter |
|
When you constructed your cubic decimeter, you were to mark the outline of a smaller cube on the lower right hand corner. What is the volume of the little cube? |
|
How many of these little cubes will fit into the cubic decimeter? |
|
Show any calculations to support your answer here.
|
|
3. Mass of one kilogram. |
|
Does the "heft" or heaviness of the mass of a kilogram surprise you? Briefly explain your answer.
|
|
Name any object that you handle regularly that has a similar mass and "heft".
|
|
4. The volume of a breath of exhaled air |
|
1. Volume of water in milliliters (m L) to fill bottle to its top |
|
Trial 1 |
|
|
Trial 2 |
|
|
Trial 3 |
|
|
Average volume of water held by bottle |
|
|
2. Volume of water left in the bottle after exhaling into bottle. |
|
Trial 1 |
|
|
Trial 2 |
|
|
Trial 3 |
|
|
Average volume of water left in bottle |
|
|
Volume of your breath calculated by subtracting the average volume of the water left in the bottle from the average total volume of the bottle. Show calculation.
|
|
Record the volume of breath you can exhale below. Post your breathe volume with your name and average volume in mL in the subject line at the appropriate site for your class section for week 2. This allows people to see the value without opening the message. This saves time. These are the WAOL embanet addresses. Use the one for your general classroom for your WAOL section. INCLUDE THE AVERAGE VOLUME OF YOUR BRETHE IN THE SUBJECT LINE. ICH-1 Week 2 WAOL WI |
|
Your average volume of breath in mL. |
|
Use six values posted by the other members of the class to calculate the average breath volume for the class in mL. Show the values you used to calculate your average. Report the average to the proper number of significant figures. Please do not give 8 digits just because your calculator generates them. Round off to the appropriate average. Example: 1100 mL + 1560 mL + 1930 mL = 4590 mL total The average volume is 1530 mL which should be rounded off to 1500 mL . This has only two significant figures. The uncertainty or variation starts in the hundreds place. |
|
Trial 1 |
|
|
Trial 2 |
|
|
Trial 3 |
|
|
Trial 4 |
|
|
Trial 5 |
|
|
Trial 6 |
|
|
Average breathe volume for six class readings in mL |
mL |
|
What is the difference between the class average and your measured volume. Give difference in milliliters and liters. |
|
|
difference in milliliters |
difference in liters. |
|
|
|
|
What is the difference as a percent of your volume? Does the percent change when you change units? Show your set up for calculations.
|
|
Give a suggestion on how you think the experiment could be improved.
Identify some aspect you liked about the experiment.
Identify something in the experiment that was new to you.
|