|
|
|
Terms: |
|
Liquid properties depend on intermolecular forces. The freezing point, boiling point, density, viscosity all are controlled by intermolecular attractions. The higher the attractions (forces) the higher the melting point, boiling point, density and viscosity.
|
|
Evaporation is a process where a liquid converts to the gas through the gradual escape of molecules from the liquid to the gas at temperatures below the boiling point. The number of escaping molecules exert a partial pressure that is less than the opposing atmospheric pressure. The molecules of the liquid will gradually escape into the atmosphere if the container is open. |
|
Vapor pressure is the gas partial pressure exerted by gas phase molecules in equilibrium with a condensed state. This means liquids and solids have vapor pressure. The equilbrium vapor pressure exists only in closed containers or systems. An open system will let gas molecules escape and the pressure they exert will be less than the equilibrium pressure. |
|
The boiling point is the temperature at which the vapor pressure equals the opposing atmospheric pressure. click to get calculator for water bp at different altitudes This means liquids can boil at almost any temperature if the opposing pressure is adjusted. When the pressure is increased the boiing point increases. Autoclaves, sterilizers and pressure cookers are sealed, elevate the pressure and raise the boiling point for a liquid. The higher the pressure exerted on the liquid the higher the boiling point for the liquid.
The boiling point can be reduced by lowering the opposing pressure. This makes cooking more difficult at high altitudes. The boiling water doesn't get as hot as it does at lower levels. Boiling an egg takes more time because the water isn't 100oC. This happens naturally at high elevations in mountain areas and cities like Denver and Mexico City. |
The normal boiling point, nbp, is the temperature at which the vapor pressure for a liquid equals 1 atmosphere. |
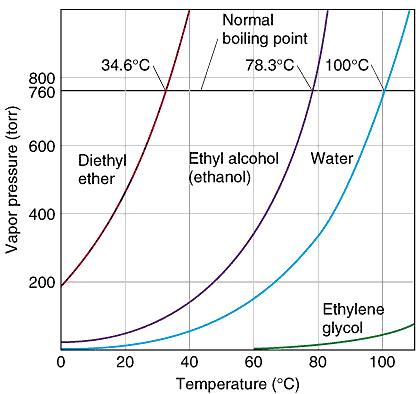
|
water H2O with a high boiling point of 100oC. This is a polar molecule with dipole-dipole forces and a great deal of hydrogen bonding between molecules. |
|
produce high normal boiling points. |
|
WHAT IS MEANT BY NORMAL BOILING POINT? answer is here |
Online Introductory Chemistry
|
|

