|
|
|
Types of solids |
|
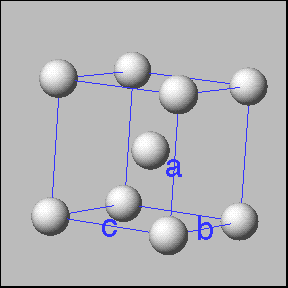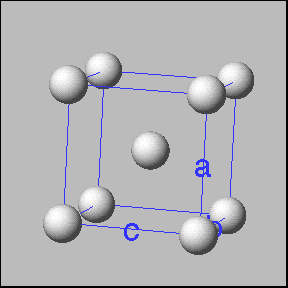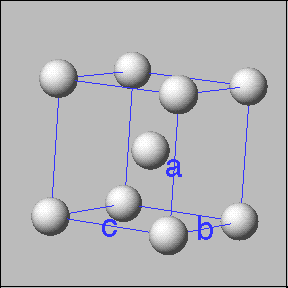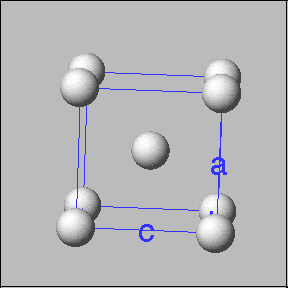
Crystalline solids have atoms, molecules or ions arranged in a regular pattern. Sodium chloride is an example of a crystalline solid. These solids have a fundamental building block called a unit cell. The unit cell consists of a set of points that mark out the positions of atoms, ions or molecules. The locations of these points can generate a complete crystal when stacked or repeated in three dimensions. |
|
Amorphous solids have no regular pattern for molecule, atom or ion locations. Glass and tar are examples of amorphous solids. These types of matrials have NO simple repeated unit cell. The structure is more complicated because they lack the repetitive units.
|
|
Examples of unit cells |
|
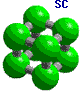
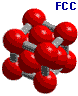
Cubic unit cells: Simple cubic -----------body centered cubic ---------face entered cubic |
|
|
Online
Introductory Chemistry
Dr. Walt Volland, All rights reserved 2000-2011 Last revised November 7, 2011