|
Kinetic theory and kinetic energy |
Kinetic
energy Kinetic energy is mechanical energy an object has because it is moving.
Any moving object can do work against anything it hits. The formula for kinetic energy gives us a way to figure the amount of work the moving object could do on impact. The total mechanical energy of an object is the sum of its kinetic energy and potential energy. When dealing with billions and billions of
molecules average values determine the outward
properties of the sample. The kinetic energy of one molecule
is not as important as the average value. Average
kinetic energy is defined by the following
equation. Here the bar over the KE indicates
average kinetic energy. The "m" is the mass in kilograms and
the "v" is the average velocity in meters / second. These units give the energy in joules. A common unit we use everyday is the watt. All of our electric appliances are labelled with their use of energy in terms of watts. A watt is power consumption that uses a joule every second. A 100 watt light bulb uses 100 joules every second. A 100 watt light bulb gets pretty hot. Note 1 joule = 1 kg m2/s2 click to see more about joules A kinetic energy calculator is available at this site. click for KE
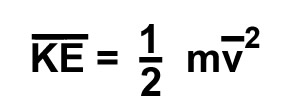
|
Kinetic molecular theory, KMT |
|
The kinetic molecular theory (KMT) is a model that does a good job of explaining the properties of matter. The KMT applies to all three states of matter but we will only describe how it applies to gases. |
|
The properties of solids, liquids and gases are consistent with this model. Different assumptions apply to the three states of matter. The solid and liquid states assume intermolecular attractions keep the particles in the condensed states. The gas state has a series of special assumptions. these assumptions make up the Kinetic-Molecular Theory of Gases. |
|
|
These assumptions are consistent with the fact that gases diffuse faster at higher temperatures. The observation that gases are compressible agrees with the assumption that gas molecules have a small volume compared to the container. Billiard ball elastic collisions agree with the observation that gases when left alone in a container do not appear to lose energy, cool and spontaneously convert to the liquid. |
|
Note The asssumptions have limitations. For example, gases can be liquified if cooled enough. This means real gas molecules do attract one another, otherwise the molecules would not stick to one another and condense. |
|
|