Solids, Liquids & Gases: States of Matter
Pressure
Pressure is a commonly used word.
It is applied to psychological stress and mechanical
conditions. Pressure has a very specific meaning in
chemistry. It is defined as force divided by area. It may be
worthwhile to recall that a force is a "push" or a "pull". Pounds per square inch is a
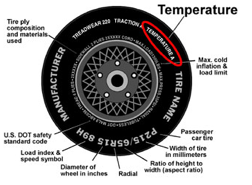
pressure unit that literally states the definition. PSI
labels are visible on inflatable objects like basket balls,
inflatable boats and car tires. Pressure unit have the qualities of
force (weight is a measure of force) in the numerator or top of the fraction and
area in the bottom. The PSI unit has pounds in the top and square inches in the denominator or bottom. . The area in square inches is related to inches x inches = inches2, in the
denominator.


|
Atmospheric Pressure |
|
When we stand on the floor, we exert a force against the floor. The pressure we exert is our body weight in pounds divided by the surface area under our feet or shoes. In snow country we may see people wear snow shoes. They do this to avoid sinking in the snow. This trick spreads a person's body weight over a larger area. The constant body weight and larger area produces a lower pressure on the snow surface. The connection between vehicle tire pressure and vehicle weight is one reason why large vehicles have big tires. The large mass must be distributed over large area or the pressures would be exceptionally high. The pressure in a truck tire is monitored for safe operation by most trucking companies. The average tire pressure in a truck tire depends on the model. It is determined by the speed at which a truck will travel, the type of load that it carries, and the conditions in which it will travel. According to the Society of Automotive Engineers the average pressure in a truck tire can range from 30 to 100 psi (pound-force per square inch) or 150 to 700 kPa (kilopascal). |
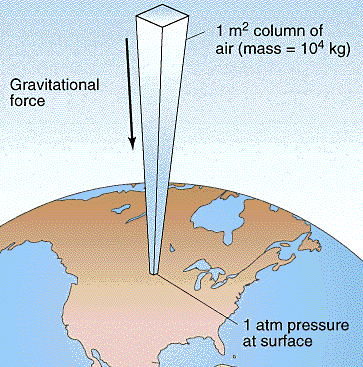
Good facts know about atmospheric pressure units.
1 atmosphere = 760 mm mercury = 29.92 inches of mercury = 101,325 Pascal
Click here for a gas pressure unit conversion calculator