Pressure units and the barometer
Commonly used pressure
units are listed here. Unit Symbol 1 atm equivalent Millimeters of mercury mm Hg 760 mm Hg Pounds per square inch PSI 14.7 PSI torr torr 760 torr Hg Pascal Pa 101,325 Pa atmospheres atm 1 atm Inches of mercury in Hg 29.92 inches Hg
Pressure
measurements A list of pressure units is good as
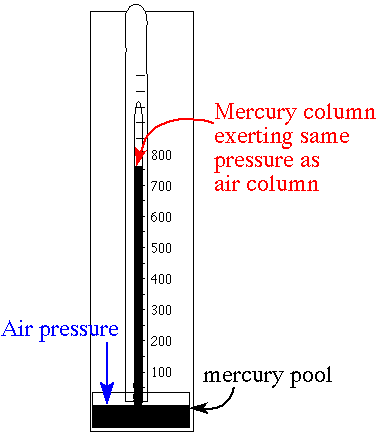
a reference but how is pressure measured. The barometer is the simplest
instrument for measuring atmospheric pressure. The earth's
atmosphere at sea level has a weight of 14.7 pounds over a
square inch of surface. This is the weight of a column of
air that extends from sea level at the earth's surface to
the edge of the atmosphere. This weight changes as the
temperature and composition of the air mass changes. The
changes are related to weather changes. A high barometer
reading indicates dense air usually associated with stable
cold weather. A low barometer reading is usually associated
with unstable weather. A barometer uses a substitute
column of mercury fluid in place of the air. A cheaper
barometer can be made using a column of water. Original
barometers where made using water. This was a nuisance
because the length of the water column is about 32 feet. for
one atmosphere. Measurements were done using ladders. More
convenient barometers can be made using mercury. Mercury is the most dense fluid
available. It has a density of 13.6 g/mL. This is 13.6 times
the density for water. A barometer using mercury is more
compact. One atmosphere in a mercury barometer is equaled by
a column of only 760 mm Hg. The column of mercury needed is
shorter when the weight of the air column decreases. This
matches "low" pressure. On the other hand when the weight of
the air column increases the length of the mercury column
increases. More mercury is needed to equalize the heavier
air column. Old time aircraft carried
barometers to measure altitude. The higher the altitude the
lower the air pressure and the shorter the column of mercury
needed to equalize the air pressure. If you go high enough
there is no air pressure to speak of the mercury column
would be zero. This decrease in pressure with increasing
altitude is one reason why commercial aircraft have
pressurized cabins. The pressure inside is maintained
artificially. The Hollywood adventure movies
showing people blown out of airliners are based on some
truth. The real fiction appears when actors are shown able
to hang on to an aircraft and breathe easily at 30,000
ft.