Pressure Measurement
|
Manometer and how
to measure gas pressure |
|
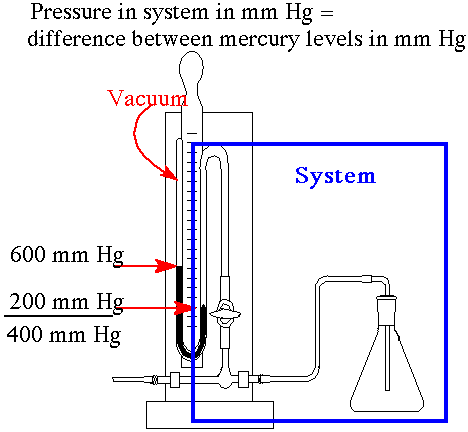
The manometer is one of the simplest tools for measuring gas pressure differences. A manometer is a u-tube. One side of the "U" is a vacuum and the other side is connected to a closed container. The "U" is filled with a fluid (usually mercury). If both sides of the "U" have the same liquid levels then the pressure inside and the pressure outside are the same. The difference between the liquid levels equals the pressure difference between inside and outside. The mercury level will be lower on the side with greater pressure. The higher pressure "pushes" the mercury down. |
|
Example: |
|
Here the gas pressure in the flask in the system is 400 mm Hg. The manometer is connected to the flask. The manometer has mercury in the U-tube. The liquid level is lower on the flask side. The liquid level is higher on the vaccuum side by 400 mm. The gas in the flask has "pushed" down the liquid on the flask side. |
|
P system = Hg level vacumm side - Hg level system side |
|
|
A good fact to know is the relationship between units for measuring pressure..
|
|
|
|