Specific gravity, temperature effect on density and use of specific gravity
|
|
The gram was once defined as the mass of a cubic centimeter of water at 4 degrees C. The density of water varies with temperature. At 4 degrees C the density is 999.9720 kg/m3 or 0.9999720 g / ml and rounded to one significant digit the density is is 1 gram per cc. Density changes with temperature because materials expand with increasing temperature. In other words volume increases when water is heated. Typically the density deceases as temperature increases. Density of liquid water
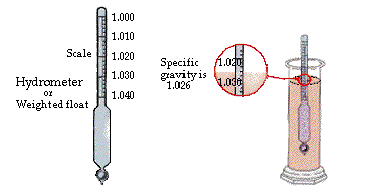
Specific gravity is a property that relates the density of materials to the density of water at the same temperature. Specific gravity has no units because it is the density of a substance divided by the density of water. The specific gravity of a substance is the same for the metric and English system of units. If a material (such as iron) is 7.85 times as heavy as an equal volume of water then it has a specific gravity of 7.85. Iron has a density of 7.85 g / ml or 7.85 kg per liter. Specific gravity = density of substance / density of water Specific gravity is particularly useful when dealing with solutions. The specific gravity can be correlated with the amount of material dissolved or suspended in a liquid. Liquids: A liquid’s specific gravity can be determined with a hydrometer. The depth to which the hydrometer sinks is inversely proportional to the liquid’s specific gravity. A hydrometer is a hollow, sealed, calibrated glass tube. The hydrometer is put in the liquid and allowed to sink. When the hydrometer stops bobbing up and down the liquid level against the hydrometer will indicate the specific gravity.
Solids: Some electronic scales can measure a solid’s specific gravity. This is particularly useful for determining a gem’s purity. Gases: Specific gravity transducers measure a gas’s specific gravity. They are particularly useful in power, oil, gas, aerospace, and process industries. Food production: Specific gravity is used to monitor the brewing of beer and the fermentation of wine. Dairy products are checked for fat content using specific gravity and egg shell specific gravity is checked in egg production. Health care: Percent body fat estimates can be made by measuring a person's weight and body volume. Adults generally have urine with a specific gravity in the range of 1.010 to 1.025. High specific gravity is seen when the body retains water and excessive solids are eliminated. There are more solutes mixed in the urine. Diuretics increase the elimination of water. The specific gravity of urine goes down as the concentration of solids is diluted. Increases in specific gravity (hypersthenuria, i.e. increased concentration of solutes in the urine) may be associated with dehydration, diarrhea, emesis, excessive sweating, urinary tract/bladder infection, glucosuria, renal artery stenosis, hepatorenal syndrome, |
| The specific gravity is read directly from the scale on the hydrometer as it floats in the liquid. The hydrometer sinks deeper in low density liquids and rides high in high density liquids. The specific gravity is between 1.010 and 1.020. |