![]()
|
|
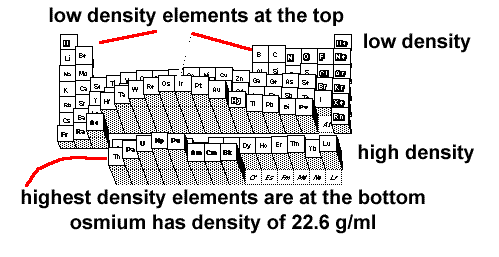
Density is a intensive property that can be used to identify elements and materials. Density is a measure of the amount of matter in a substance. It is defined as the mass of an object divided by its volume. D = mass / volume Under normal conditions densities fall within a narrow range. The most dense element is Osmium. It has a density of 22.6 grams / ml. The lowest density for an element is recorded for hydrogen gas. Hydrogen (H2) gas at standard conditions has a density of 0.00008988 grams per ml. Gas densities are so small that they are often listed in grams per liter. This makes the hydrogen gas density value 0.08988 g/ liter. The label heavy metal elements is justified for the metals in the sixth row of the periodic table. These elements platinum, Pt; mercury, Hg; gold, Au; iridium, Ir; and tungsten, W have some of the highest observed densities for materials at normal conditions.
Gold is very dense. It has a density of 19.32 grams per ml . It is 19 times more dense than water. To put this in perspective a gallon of water has a mass of about 8.3 pounds. The gallon container filled with gold would weigh 160 pounds. A five gallon jug filled with gold would weigh 800 pounds. Many cars have 18 gallon gas tanks. If the gas tank was filled wth gold it would weigh almost 3000 pounds. A bank once had a cubic foot of gold in its vault. It offered to give it away to anyone who could lift it and carry it out of the bank building. A cubic foot of gold weighs about 1200 pounds. The gold stayed put and there were no takers of the offer. ( another pun? ) 1. Which element has the greater density gold or platimum? 2. What is the trend in density going from top to bottom of a group? 3. Which element has the higher density helium, He or radon, Rn? 4. Lead, Pb, has a density of 11 g/ml. Which metal magnesium, Mg, or zinc, Zn has a density closer to that for lead? Answers: 1. Gold has a greater density than copper. Gold prospectors panned for gold by looking for the high density gold nuggets at the bottom of their pans. 2. Density increases going from the top of a group to the bottom. 3. Radon at the bottom of Group 8A has a greater density than helium at the top of Group 8A 4. Zinc is closer to lead in the periodic table. Zinc has a higher density than magnesium so it is closer to lead. |