Online Introductory Chemistry
|
|
Self test on atoms and the periodic table
|
1. |
What is the number of electrons, protons and neutrons in an atom of radon-224? Identify the block that contains radon. |
|||||||||
|
2. |
Copper has two naturally occuring isotopes. What is the average atomic mass for copper? Use the percent abundances and masses given here.
|
|
3. |
Locate the following on the outline of a periodic table. a. alkali metals b. halogens c. elements with an "ns2np3 " set of outer electrons. Explain how to locate elements with this outer electron set. |
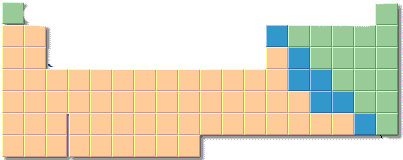
|
|
4. |
Write the electron configuration for an atom with 17 electrons. Mark the valence electrons. What is the identity and group for this element? Tell whether this element is a metal, nonmetal or semimetal. Explain how each of these types of elements differ. |
|
5. |
Sketch an atom of berylium-9. Indicate the number of neutrons and protons in the nucleus. Show the relative average distances out from the nucleus for the electron subshells. Identify the valence electrons. Tell whether or not this element is an "s-block" element. Explain what it means when an element is in the "s-block". |