Online
Introductory Chemistry
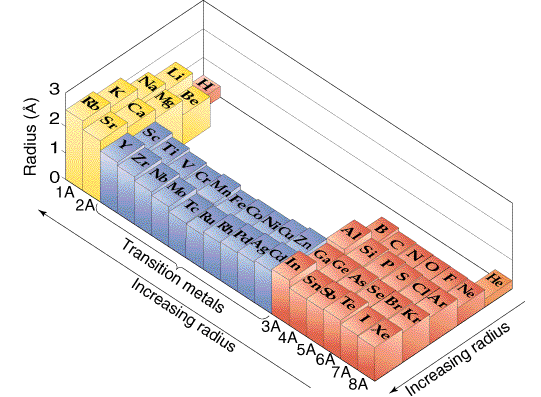
Electron Configuration, Block classifications, Atomic radius trends and the Periodic Table
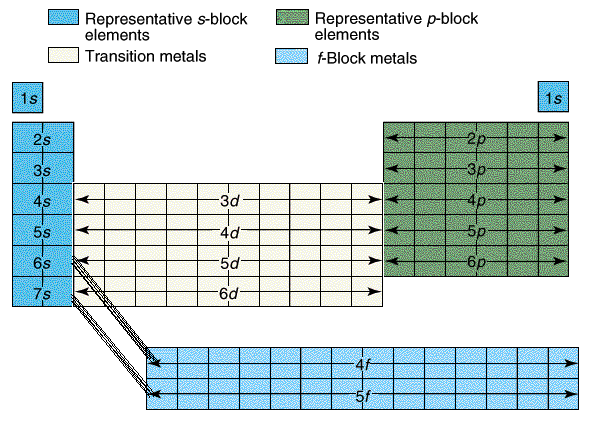
The periodic table shape results from the build up of electrons in the shells and subshells of the atoms as the atomic number increases. Every step up in atomic number (a proton is added to nucleus) requires an additional electron to keep the atom neutral. The periodic table has a series of BLOCKS. One BLOCK is made of 2 columns, a second BLOCK is made of 10 columns, a third BLOCK is made of 6 columns and fourth BLOCK is made of 14 columns. The BLOCK sizes match the number of electrons that fit into the s, p, d and f subshells. The s subshell can hold two electrons. This matches the size of the s-block. The p subshell can hold up to six electrons. This matches the size of the p-block. The d subshell can hold up to 10 electrons. This matches the size of the d-block with 10 columns. The f subshell can hold up to 14 electrons. The 14 columns of the f-block match the filling of the f subshell. |
Note that the 4f-block elements appear between the 6s and 5d block.