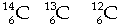Online Introductory Chemistry
Isotope definition and average atomic masses
|
|
| Isotopes: |
Greek "iso" means same and "topos" means place. The periodic table has atoms with the same atomic number but different atomic weights in the same place in the table. All the atoms of an element have the same atomic number (number of protons), but they can have different numbers of neutrons and different masses. click for more We say that isotopes of an element are atoms that have the same number of protons, but different numbers of neutrons. An example of this is shown with the three different isotopes of carbon. Here they all have 6 protons, but different numbers of neutrons. Carbon-14 has 8 neutrons. Carbon-13 has 7 neutrons and carbon-12 has 6 neutrons. Carbon-12 is the most common isotope of carbon. Carbon-14 is radioactive and very rare.
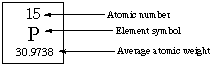
The symbols for the isotopes of carbon atoms shown here indicate they each have six protons but mass numbers of 14, 13 and 12. These isotopes are not equally abundant. Carbon-12 (C-12) makes up 98.9 % of all carbon atoms while carbon-13 (C-13) makes up 1.1 %. Carbon-14 is very rare, not stable and is radioactive. The periodic table location for all the isotopes of an element is the same. This fits with the Greek "iso" means same and "topos" means place. Periodic table entries typically provide atomic number, average atomic mass and the element symbol. This is the information shown here. Here we see that phosphorus has the symbol "P" . It has an atomic number of 15 because the nucleus has 15 protons. The average atomic weight or mass is 30.9738 atomic mass units or amus. The periodic table does not usually indicate isotope information.
|
|
Isotope abundances: The isotopes of an element do not occur with equal frequency or amounts. click here for table of abundances of stable isotopes. A mass spectrometer is able to separate isotopes and provide measurements about the abundances and types of isotopes for elements. click here for more information. The three isotopes for carbon do not occur equally in nature. The relative abundance depends on the relative stability of the isotope. The relative abundances for the three carbon isotopes are carbon-12 is 98.9%, carbon-13 is 1.1% and carbon-14 less than .001%. The isotopes contribute to the average atomic mass based on their abundance. The result is that the "average" mass for the atoms of an element is dictated by the most abundant or common isotope. The average atomic mass for carbon is 12.0107 amu. Because the atomic weights in the periodic table are weighted averages the tabulated atomic weight value doesn't match any actual atom, but is closer to the weight for the most common isotope. The weighted average is calculated in the same way you figure out your grade after a series of assignments. An example calculation is on the next page. |