


Online
Introductory Chemistry
Isotopes and average atomic masses
|
Dr.
Walt Volland revised July 5, 2010
|
- Example
of a weighted average calculation:
-
- Assume
you have taken five quizzes with the following scores out
of 10 possible points. 10 pts, 5 pts, 10 pts, 10 pts, 10
pts. The average score will be 9 pts.
|
-
average
= (1/5)( 10 pts + 5 pts + 10 pts + 10 pts + 10 pts) =
45 points total / 5 = 9 pts
|
The low score doesn't
shift the average down much because the four 10 point scores weight
the average toward nine. Here the high scores occurred 4 out of 5
times or 80% of the time. Only one out of five scores was low at 5.
The abundant high scores have more influence on the average. You can
calculate the weighted average in the following way. Literally what
happens in the calculation is
-
average
score = [40 points + 5 points][1/5] =
[45 points] = 9 points
|
|
| Example
weighted average calculation using isotope abundances: |
-
What is the
average atomic weight for chlorine if it has two isotopes? The percent
abundance for chlorine-35 is 75.53%. The percent abundance
for chlorine-37 is 24.4%. The mass for Cl-35 is 35.0 amu and for Cl-37
it is 37.0 amu.
|
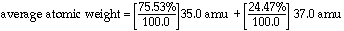
Average
atomic weight = 26.4 amu + 9.05 amu = 35.5 amu
|
Notice
that the average is determined by the more abundant Chlorine-35.
Because chlorine-35 is more common, the average is closer to 35
amu than 37 amu. Clearly no atoms of chlorine actually have
a mass of 35.5 amu. The tabulated value in the periodic
table is a statistical creation and matches no real chlorine atoms at all.
|
| Exercise: |
| What
is the average atomic mass for thallium, Tl? The two stable isotopes and their abundances are listed here.Tl-205
has a mass of 205.059 amu with an abundance of 70.48 % and Tl-203 has
a mass of 203.059 amu with an abundance of 29.52 % |
1. Convert percentages to decimals
29.52 % to 0.2952 for thallium-203
70.48 % to 0.7048 for thallium-205 |
2. The general formula used is:
weighted average = ( decimal fraction A) mass A + ( decimal fraction B) mass B
|
3. decimal fraction A = 0.2952 ; decimal fraction B = 0.7048
|
4. mass A = 203.059 amu ; mass B = 205.059 amu |
| 5. Weighted average = 0.2952 x ( 203.059 amu) + 0.7048 x ( 205.059 amu) = 204.466 amu |
- 6. Answer:
- The percentages have only four sigificant figures. The average needs to be rounded to four sf.
- The result 204.466 amu when rounded off gives 204.5 amu with 4 significant figures.
|



![]()