Online Introductory Chemistry
How to predict electron configurations
|
|
Online Introductory Chemistry
How to predict electron configurations
|
|
|
Predicting electron configurations and summary of order of filling of subshells: method 1 |
|
Predicting electron configurations diagram: method 2 |
|
This triangle can be built and used to predict the electron configuration for most any atom. Each successive row has an n value larger by one unit. You should note that there is one more subshell added when the "n" value increases by one. The top of the diagram matches the top of the periodic table. Every column has the same subshell type. |
|
|
Population limits for subshells |
|
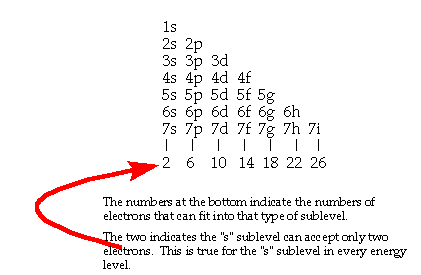
|
Electron configurations using the diagram method EXAMPLE |
|
This triangle can be built and used to predict the electron configuration for most any atom. Here the electron configuration for Li is figured out. The electron configuration for lithium uses only the 1s and 2s subshells. The third electron in lithium has to go into the second level because the 1s is completely filled after two electrons occupy the level. You try doing the electron configuration for boron, B, with five electrons.Click here to see the answer What is the electron configuration for magnesium atomic number 12? Click here to see answer. |
|