Online
Introductory Chemistry
The Periodic Table and Electron Configurations
revised July 5, 2010 all rights reserved
Online
Introductory Chemistry
The Periodic Table and Electron Configurations
revised July 5, 2010 all rights reserved
|
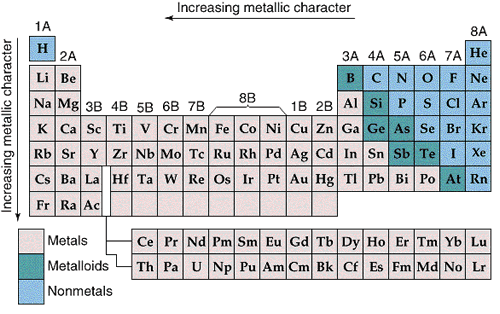
A period in the periodic table is a horizontal row of elements with outer electrons in s and p subshells in the same shell. For example all the elements in row two of the periodic table have outer electrons in the n = 2 shell. The animation below shows how electrons are filling the second shell for the elements Li through Ne in the second row. All the second period elements have the same 1s2 or [He] electron core. Every element in a row of the periodic table has the same number of "core" electrons. The animation below shows the electron configurations for the row two elements with a helium core, [He] of 1s2. click here to see electron configurations for all the elements.
Typically the core electrons are in the filled s, p and d subshells. The core electrons are the electrons closer to the nucleus and are not "valence" or outer electrons. The core electrons for row three are the ten electrons in the set 1s22s22p6 or [Ne] core. The core for row four is made up of the eighteen electrons in 1s22s22p63s23p6 or the [Ar] core. The rare gas elements in Group 8A have an electron count that matches the core for the elements in the 'following' row of the table. |
|
A group in the periodic table is a vertical collection of elements with the same number of outer electrons. They all have similar chemical properties because they tend to lose or gain the same number of electrons in reactions. These kinds of similarities are strongest in the alkali metals in Group 1A and in the halogens in Group 7A. |
|
The main group elements are in the "A" groups. There are eight main groups from 1A through 8A. The main groups elements also start at the top or first period in the table. The main group elements have outer electrons in the nsnp subshells where n equals the row number in the periodic table. A nice thing about this numbering system is that the group number indicates the number of valence ( outer ) electrons for atoms in the group. In our class we focus on the Representative or main group elements. Example: Sodium, Na, is in row three and in Group 1A. Sodium with the electron configuration 1s22s22p63s1 has one outer electron and ten core electrons the [Ne] core. The outer electron is in the 3s subshell, n = 3. The electron configuration for this is [Ne] 3s1 where the outer electron is the 3s1electron. Example: Boron, B, is in row 2 and in Group 3A. Boron has three outer electrons with the electron configuration 1s22s22p1 or [He]2s22p1. These three outer electrons are in the 2s and 2p subshells, with n = 2. The outer electrons are 2s22p1 Example: Chlorine, Cl, is in row three and in Group 7A (or 17A). Chlorine has ten core electrons and seven outer electrons with the electron configuration 1s22s22p63s23p5 or [Ne]3s23p5 . The seven outer electrons are in the 3s and 3p subshells, n = 3. The outer electron distribution is 3s23p5. |
|
Why there are ten groups for the transition elements? The transition elements are in the "B" groups.
These groups are identified with the ten group numbers 3 through 12. The first transition elements appear in the third row or period of the table. There are ten different vertical columns in the transition elements. This results from the fact that there are ten spaces for electrons in the "d" subshells. These elements generally have outer electrons entering the (n -1)d subshell. Here the "n" is the row number. For example scandium, Sc, is in row 4 so the n - 1 rule gives n - 1 = 4 - 1 = 3. Scandium has 21 electrons with the last electron or 21st electron in the 3d subshell. It has a 'group' number of 3. Scandium has 3 outer electrons. |
|
Why there are eight groups for the representative elements? The periods of the periodic table are eight elements long because they match the filling of the eight spaces for electrons in the ns and np subshells, ns2np6. The "s" subshell can hold two electrons while the "p" subshell can hold six electrons for a total of eight. |