Molecular and Structural Formulas
| A molecular
formula gives the types and the count of atoms for each element in a compound.
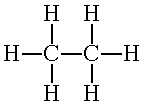
An example of a molecular formula is ethane, C2H6.
Here the formula indicates carbon and hydrogen are combined in ethane. The
subscripts tell us that there are 2 carbon atoms and 6 hydrogen atoms in
a formula unit. Both of the carbon atoms are considered to be "central
atoms" for constructing Lewis diagrams.
|
|
Lewis dot structure, C2H6
|
|
|
Lewis dot structure for water H2O Electron pairs that are shared are physically between the symbols for the atoms. Electron pairs that are unshared are called lone pairs. Lone
pairs are electron pairs not shared between atom symbols.
|

|