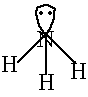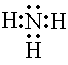Co-ordinate
Covalent Bonds
| A
coordinate covalent bond is special because it involves a shared pair
of electrons that comes from a single atom. Ammonia had a nitrogen atom
with an unshared pair of electrons. These can be shared with an electron
defficient atom like H+.The ammonium ion is formed when a H+
ion coordinately bonds to the NH3 molecule |
|
ammonia
|
ammonia
|
proton
|
ammonium
ion
|
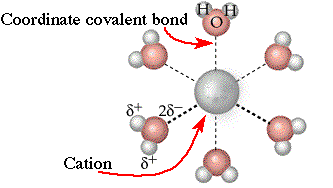
| Water
molecules have two unshared pairs of electrons. These form coordinate
covalent bonds with cations that are dissolved in water. This is one reason
why water dissolves many ionic solids. The energy released when the water
molecules bond to the cations is often enough to break up the ionic solid.
|
|
water
|
cation
|
water and cation
|
| A
dissolved cation will form as many as six coordinate covalent bonds with
water molecules. This helps account for the solubility of cations.return
to top of page |