Multiple Bonds and bond length
|
Multiple bonds are very common in carbon compounds. The carbon atom can form four bonds. These four bonds can be all single, CH3CH3, two single and one double, CH2CH2, two double, one single and one triple, CHCH. Organic compounds with carbon-carbon multiple bonds ( double and triple bonds ) are labeled unsaturated. Molecules with no carbon-carbon multiple bonds and only carbon-carbon single bonds are labeled saturated. These terms are often seen in reference to fatty acids. Hydrogenated oils are converted from unsaturated molecules with multiple bonds to saturated or partially saturated molecules. The oils are converted to solids or semisolids after hydrogenation. More information is availabe at this page |
|
Ethane has a single bond carbon-carbon bond |
|
|
|
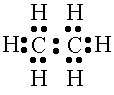
|
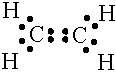
|
|
|
|
|
|
|
The carbon-carbon single bond has a bond length of 154 picometers. |
The carbon-carbon double bond has a bond length of 133 picometers. |
The carbon-carbon triple bond has a bond length of 120 picometers. |
|
The carbon-carbon bond lengths decrease as the number of shared electrons and bonds increase. This is reasonable because there are more electrons attracted to each nucleus. The repulsions between positive nuclei decrease as more electrons are shared. The relative lengths for the three types of bonds are summarized here. REMEMBER these compare bonds between pairs of atoms like ; |
|
|