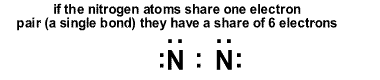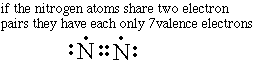Multiple Covalent
Bonds
Why
multiple bonds are formed
Why multiple bonds form: Nonmetal atoms bond to reach a stable or low energy condition. This happens when a main-group atom shares enough electrons to achieve a rare gas valence shell (usually an octet). Sometimes the number of electrons needed cannot be provided by sharing electrons simply in single bonds (pairs). Nitrogen molecules are an example of this. Nitrogen atoms have five valence electrons. We know that molecules of N2 exist based on a great deal of measurements. If two nitrogen atoms simply formed a single bond the dot structure would look like the illustraton below. Each nitrogen atom would have only six electrons not an octet. The single bond doesn't provide the two nitrogen atoms with an octet. If the octet rule is to be followed, the nitrogen atoms must share more than two electrons.
The trial and error method is used to decide how many shared electrons are needed to create a structure where the octet rule is met. Since one bond didn't work the next thing to try is a double bond where the atoms share four electrons.Unfortunately the double bond structure only provides 7 valence shell electrons not eight.
Because the single
and double bonds didn't do the trick the next thing to try is a triple
bond where the nitrogen atoms share six electrons. The count for both
atoms when a triple bond is used in the structure shows that the octet
rule is met.
Exercise: What would be the Lewis structure for carbon dioxide, CO2? The carbon atom has four valence electrons and the oxygen has six valence electrons. Click for answer |