Drawing Lewis Structures
Drawing Lewis Structures
|
Demonstration of process: We will draw the Lewis structure for the carbonate ion, CO32- |
|
|
Step 2:
Calculate
the total number of valence electrons contributed by all the atoms.
|
|
Step 3:
Check
to see if there is a charge on the formula unit. Adjust the total
in Step 2 by the number of electrons added or removed because
of any electrical charge. This gives you the final total of valence
electrons that are available to hold the particle together. total of 4 (carbon)+(3x6)(oxygen)+2(negative charge)=24 valence electrons. |
|
Step 4:
Identify central atom/atoms in the formula unit. |
|
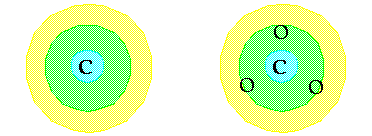
Step 5:
Think
of your structure as a target. Write down the symbol for the central
atom in the "bullseye". Then arrange the symbols for the oxygen
atoms evenly in the next ring around the center atom. |
|
|
|
Step 6:
Divide the total number of valence electrons for the formula unit
(Step 3) by 2 so you know the number of "electron pairs" for the
particle. For example, in carbonate ion, Steps 1, 2, and 3 would tell you that the molecule contains 24 valence electrons. Therefore its bond, octets, and duets are done with 12 pairs of electrons. |
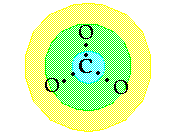
Step 7:
Connect the center atom with a single bond (1 pair
of electrons) to each neighboring atom in the second ring. Then,
using a single bond, connect each atom in the second ring to its
neighbor in the third ring. Count the number of electron pairs
used.
For example, in carbonate
ion you would connect the C to each of the O's. This would use a total
of 3 electron pairs.
Step
8: Subtract the number of electron pairs used in
Step 7 from the total number of electron pairs for the particle
(Step 6). Use these remaining electron pairs to fill out the
octets for atoms in the second ring. Step
9: The almost last step is verification that
the atoms each have an octet or duet. If they do you are finished.
If the central
atom lacks an octet when only single bonds are used you need
to move a lone electron pair to make one or more multiple bonds.
This may mean double or triple bonds as appropriate. NOTE: Any
lone pair can be used here. The negative two
charge is spread over the entire ion.
For example, in
carbonate ion you used 3 pairs of electrons in Step 7, leaving
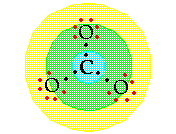
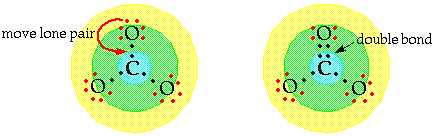
![]()