Predicting Shapes of Molecules using VSEPR Theory
Methane and sample Exercise
|
|
The valence shell electron-pair repulsion model, VSEPR, model attempts to predict molecular shapes. The model assumes that electron pairs around a central atom repel one another. Repulsions between the electron pairs force definite positions based on the number of "groups" or electron clouds. The model counts groups of electrons radiating out from a "central atom" . You can use the model to make reasonably accurate predictions. We will deal only with central atoms that have either two, three or four groups of electrons radiating from the central atom.
The three step process of determining the shape of a molecule is illustrated below.
|
|||
|
|
||||
|
|
||||
|
|
||||
|
|
The
three types of basic structures are shown here. NOTE
the electron pairs are also equivalent
to "groups" of electrons such as lone pairs,
double bonds and triple bonds. The central atoms shown here are the building
blocks for huge molecules. The angles and shapes in DNA can be predicted
using the same VSEPR style model. The basic shapes for the electron clouds
are linear, triangular planar, and tetrahedral. Note the bond angles,
actual angles will be slightly diferent because of properties of the atoms
in the molecule. The actual angles will be reasonably close. This is an
only an approximate model.
NOTE electron pairs and cloud groups are used to mean the same thing. 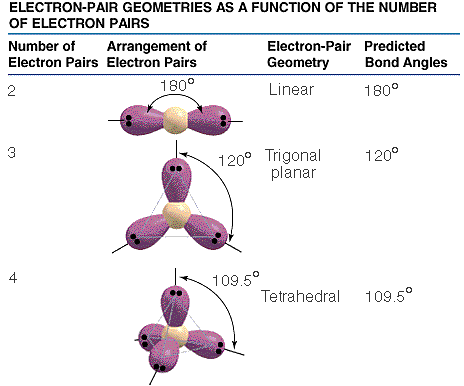
|
|||
|
|
Exercise: What is the VSEPR category for BeH2 and HCN? Draw the Lewis structure and predict shape and the bond angles. |