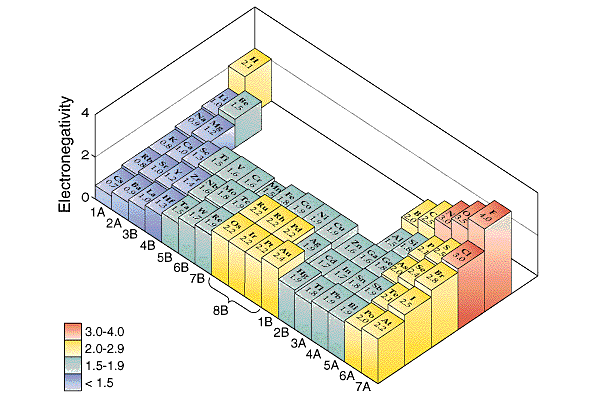
Sorry if you picked a.
The cesium is a nonmetal and
as far from fluorine as an element can be. The
bond is ionic. Great if
you picked b.
The boron and At are both
four atoms away from fluorine. The EN values are
boron, 2.0, and astatine, At, 2.2. The
electronegativity difference is very small. The
bond is slightly polar. Sorry if you picked c.
Hydrogen is a bad acting
element. It really has an electronegativity that
would put it at the bottom of group 7A. The bond
between hydrogen and oxygen is one of the most
polar covalent bonds possible. They have very
different electronegativity, EN, values.
Actually hydrogen has EN = 2.1. Oxygen is second
only to fluorine in the electronegativity scale.
Oxygen has EN = 3.5. The values are different by
1.4 units. The bond is very polar.