|
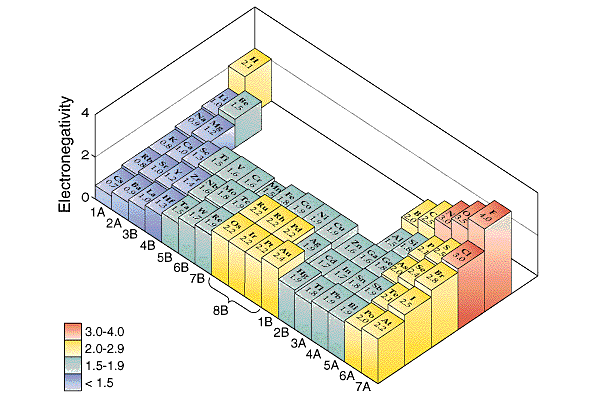
Electronegativity,
EN, is an index that tells the relative attraction an element has for
electrons in a bond. Electronegativity has a high value of 4.0 for F,
fluorine. The lowest electronegativity value is about 0.7 for Cs, cesium.
The table below shows the nonmetals have relatively high electronegativities.
The metals have relatively low electronegativities. The electronegativities
follow the same trends as atomic sizes (radius). Electronegativity gets
smaller with increasing distance from fluorine. Atoms that are equidistant
from fluorine have similar (not identical) electronegativities. The
rare gases generally are not tabulated for EN values.
Bond
polarity is directly related to electronegativity difference. The greater
the difference in electronegativity the more polar the bond.
When
two elements are next to one another in the periodic table they have
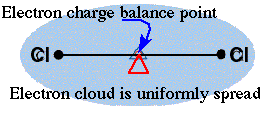
similar electronegativities. Chlorine has a value of 3.0 while bromine
has a value of 2.8. These two atoms in BrCl would have a nonpolar covalent
bond.
The
bond between carbon with 2.5 and nitrogen with 3.0 would be polar. The
bond between chlorine, 3.0, and boron 2.0, would be polar. When electronegativities
get too different the bond is not polar, it is ionic. The nonmetal gets
the electrons from the metal essentially 100% of the time. The metal
atom is stripped of its valence electrons. These atoms don't share the
electrons. There is a transfer to the nonmetal.
Differences
in EN are related to positions in the periodic table. The greater the
separation between the elements the greater the electronegativity difference.
Look at fluorine and cesium. This is the greatest difference possible,
4.0 - 0.7 = 3.3. This combination is so different that they form ionic
bonds.
hydrogen really fits in best at the bottom of the halogens,
group 7A.
|