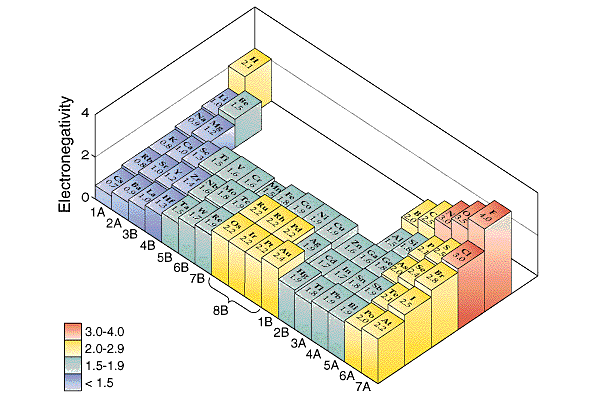
Sorry
if you picked a. The chlorine is one atom away from fluorine.
The boron is four atoms away from fluorine. The fact that
chlorine is less elecronegatie than fluorine makes the B-Cl
bond less polar than the B-F bond. and boron are both Cl and
B Great
if you picked b. The fluorine and boron have the biggest difference
in EN so they would have the most polar bond. Sorry
if you picked c. Carbon
and iodine are two and three atoms away from fluorine. They
have similar electronegativity,EN, values. Actually carbon
has EN = 2.5 and Iodine has EN = 2.5. the values are identical.
RETURN
TO LESSON