Polar Molecular Compounds and Dipoles
|
The term DIPOLE describes a molecule that has a positive pole and a negative pole. The molecule has a relatively POSITIVE CENTER and a RELATIVELY NEGATIVE CENTER. These two centers form the POLES. They are at different parts of the molecule structure. Polar molecules line up in the presence of an electric field. The positive ends of the molecules are attracted to the negative portion of the electric field. The negative end of the molecules are attracted to the positive part of the electric field. In the picture below the RED positive electrical surface in red attracts the negative end of the polar molecule. The BLUE negative charged electrical surface attracts the positive end of the polar molecule. The polar molecules LINE UP in the space between the electrically charged plates or surfaces.
The electronegativity, EN, is an index for the ability of an atom to attract electrons. Metals have low EN values and nonmetals have large electronegativity values. Fluorine, F,has the highest EN of 4.0. The element with the lowest EN is lithium, Cs, with a value of 0.7. Fluorine is at the top of Group 7A. Cesium is at the bottom of Group 1A. The electronegativity, EN, of the atoms in a bond must be compared to determine whether or not the bond is polar. The periodic table shown here has electronegativity values, EN, for the common elements. The lower limit for a polar bond is an electronegativity difference of about 0.4 electronegativity units. This means a bond between carbon with a electronegativity of 2.5 and nitrogen with an electronegativity of 3.0 will be polar.
The HCl molecule has a polar bond between the H atom and the Cl atom. There is a large electronegativity difference between the two atoms.
This is more than the 0.5 minimum needed for a polar bond. The linear molecule has a relatively + charge at the hydrogen end and a relatively negative - charge at the chlorine end. The polarity of bonds is indicated by an arrow. The arrow points at the more electronegative atom the pair. The more different the electronegativity the more polar the bond.
Symmetric molecules are usually nonpolar. The polarity marked by the arrows all cancel out. The sum of all the little arrows may cancel out as in carbon dioxide. The CO2 molecule is nonpolar. When the arrows do not cancel the molecule is polar as in water. Asymmetric molecules are polar if there are polar bonds in the structure. .
|
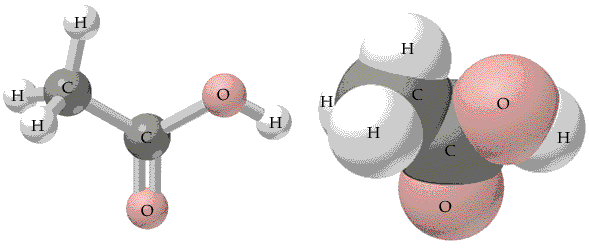
Acetic
acid has the structure shown below. The oxygen end of the molecule is
slightly negative . The molecule is not symmetric. It has a negative
end where the oxygens are. The other end of the molecule is slightly
positive.