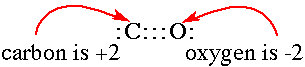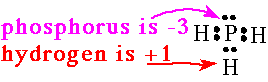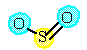|
revised October 12, 2009 |
|
Acids, Bases, Neutralization, Net Ionic Equations Oxidation numbers and Redox Reactions |
|
Acids are defined as H+ protons donors. These are the common binary acids (in pink) and typically are dissolved in water.
The pure HX compounds are not considered to be acids. The pure materials are gases at room temperature except for water.
|
|
Bases are hydroxide donors. These compounds a a combination of a metal ion and the hydroxide ion, OH1-. Magnesium hydroxide , Mg(OH)2, is a common base.
|
|
Metal oxides like MgO and Na2O are called basic oxides, because these compounds react with water to form hydroxide compounds. |
|
|
|
Neutralization is the process of reacting an acid with a base. |
|
|
|
The idea is that the reaction "neutralizes" the protons from the acid and the hydroxide from the base. |
|
There are compounds like carbonates, Na2CO3, that do not contain hydroxide in the formula but on contact with water form hydroxide ions, OH1-. |
|
|
|
Exercise: What is the acid in the reaction? What is the coefficient needed to balance the equation? |
|
|
|
This is a very involved concept. It requires knowledge of the solubility and ionization properties of reactants. The value of the concept lies in the idea that reactions are written showing only ions and compounds that "change" in the reaction. Reactions that occur in water solutions do not really happen between the formula units for the reactants. The process involves the dissolving process first. When "soluble" materials dissolve the formula unit breaks into ions. Example:
|
|
Spectator ions are ions that remain unchanged in the reaction. A net ionic equation is written showing only the ions and formulas that actually change in the reaction.
|
|
Acid base reactions are identified by the formation of water when a hydroxide ion and a proton combine. The apparent charges on ions and atoms stay the same, hydrogen stays +1 and oxygen stays -2. Oxidation reduction reactions are different. One substance is oxidized and the other is reduced. The difference is that electrons are transferred in the oxidation reduction reaction.
Oxidation
is the loss of electrons Zn(s)
---> Zn2+(aq) + 2 e1- Reduction
is the gain of electrons Cl2
(g) + 2 e1- ----> 2 Cl1-(aq)
The number of electrons lost by the oxidized atom equals the number of electrons gained by the reduced atom. The atoms have a change in oxidation number. The oxidation number is the charge on an atom. |
|
A general rule about redox reactions is that if an atom or formula gains "O" oxygen it is oxidized.return to top of page If a formula or atom loses oxygen it is reduced. Hydrogen can be used as a "screening" tool . An atom or formula that gains "H" hydrogen is reduced and any formula or atom that loses hydrogen is oxidized. |
|
1. Atoms in pure elements have an oxidation number of "0".
|
|
2. Atoms in monoatomic ions have oxidation numbers equal to the charge on the ion. return to top of page |
|
|
3. The more electronegtive atom in a bonded pair has a negative oxidation number. In binary molecules the more electronegative atom has the same number it would have as a simple anion. |
|
|
|
4. The sum for all the oxidation numbers add to zero for neutral formulas.return to top of page |
|
|
Example of redox reaction. return to top of page |
|
The reaction of oxygen with ethane is shown here. |
|
|
|
The "O" is reduced. It gains hydrogen. The CH3CH3 is oxidized, it gains "O" and loses "H" |
|
|
|
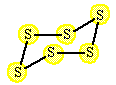
Example: The reaction of oxygen with sulfur is an oxidation reduction reaction. |
|
|
|
|
|
|
|
|
|
|
|
Oxygen has 0 |
reduced |
Oxygen has -2 oxidation number |
|
Sulfur as the pure element started with "0" oxidation number. It is oxidized to sulfur with +4 oxidation number. return to top of page |