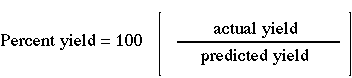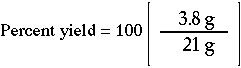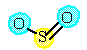|
Percent Yield Definition and Example revised October 12, 2009 all rights reserved Dr. Walt Volland 2005-2009
|
|
The percent yield is defined as 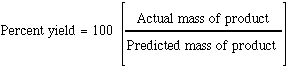 return
to top of page
return
to top of page
|
|
The predicted yield is determined by the masses used in a reaction and the mole ratios in the balanced equation. This predicted yield is the "ideal". It is not always possible to get this amount of product. Reactions are not always simple. There often are competing reactions. For example, if you burn carbon in air you can get carbon dioxide and carbon monoxide formed. The two reactions occur simultaneously. Some carbon atoms end up in CO and others end up in CO2. The typical calculation in a starting class assumes that there is only one path for the reactants. This is an over simplification.You know for example from real life that food is not always converted to energy. If you eat a cookie, some of it could end up stored as "fat" Ugh!
|
|
What is the percent yield for a reaction if you predicted the formation of 21. grams of C6H12 and actually recovered only 3.8 grams? |
|
1. Recall definition of percent yield. |
|
|
2. Substitute the actual and predicted yields. |
|
3. Answer: The percent yield is 18 %.
|
|
Example: |
|
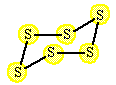
A reaction between solid sulfur and oxygen produces sulfur dioxide. |
|
The reaction started with 384 grams of S6 (s). Assume an unlimited supply of oxygen. What is the predicted yield and the percent yield if only 680 grams of sulfur dioxide are produced? |
|
1 S6 (s) |
6 O2 (g) |
|
6 SO2 (g) |
|
|
|
|
|
|
Step 1 : Calculate the molar masses for S6 (s) and SO2(g). The oxygen has no effect on the answer because there is more than you need. 1 mole S6 (s)= 193 grams S6 (s); 1 mole SO2(g) = 64 grams SO2(g) |
|
Step 2 : Mole ration method Determine the mole ratio for 1 mole S6 (s) to mole SO2(g) The balanced equation indicates 1 mole S6 (s) to 6 mole SO2(g) |
|
Step 3: Calculate the number of moles of S6 (s) moles S6 (s) = [384 g S6 (s)][ 1 mole S6 (s)/ 192 g S6 (s)] = 2 moles S6 (s) |
|
Step 4: Calculate the moles of SO2(g) expected using the mole ratio 6 moles SO2(g) / 1 mole S6 (s) moles SO2(g) = 2 moles S6 (s)[6 SO2(g) / 1 S6 (s)] = 12 moles SO2(g) |
|
Step 5: Calculate the grams of SO2(g) predicted using 1 mole SO2(g) = 64 grams SO2(g) grams SO2(g) = 12 moles SO2(g)[64 grams SO2(g)/1 mole SO2(g)] = 768 g SO2(g) |
|
Step 6: Calculate the percent yield using the definition Percent yield = 100[actual yield/ predicted yield] = 100[680 grams SO2(g)/ 768 g SO2(g)]= 89% return to top of page |
|
|
|
|