Self Test on rates of reaction, equilibrium expressions, equilibrium constants, Le Chatelier's principle
Short
essay questions
1. What is a reversible
reaction? 2. Sketch the plot
of reaction rate versus extent of reaction for reactants and products. Describe how the
rate for the forward reaction changes with time. Describe how the
rate for the reverse reaction changes with time.
3. Write the equilibrium
expressions for the following equations. Explain
4. Which of the following
reactions has a positive entropy change and which has a negative one? Justify your
answer.
5. A reaction has a
DH = +5600 calories. The entropy change is -4.6 calories /Kelvin at
298 Kelvin. Is this
reaction spontaneous? Will raising the temperature ever make it spontaneous?
6. An equilibrium reaction
has the following equilibrium expression. Assume DH = -75,600 calories.
What is the effect
on [SO2 ] if the pressure is increased? What happens
to [SO2 ] if the temperature is raised?
.
7. Give definitions
for activation energy, heat of reaction, endothermic, exothermic, spontaneous
reaction. What is the standard used for predicting spontaneous reactions? What effect does
temperature have on reaction rates? Can an activation energy ever be
negative? Explain your answer.
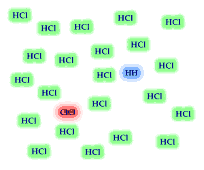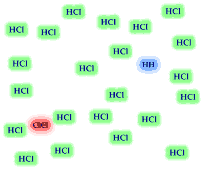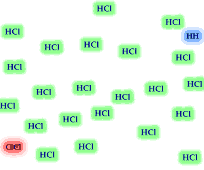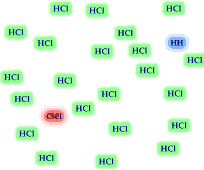
8. The reversible reaction
H2 (g) + Cl2 (g) <----> 2 HCl(g)
has the equilbrium expression The equilibrium
mixture of reactants and products is illustrated. Based on the illustration
which side of the equilibrium is favored, reactants or products? Is the value for
the equilibrium constant greater than "1"? If you added 24
H2 and 24 Cl2 molecules to an empty container,
how many H2 and Cl2 molecules would remain when
the reaction reached equilibrium? Would the HCl molecules out number
the Cl2 and H2 molecules?
Multiple choice 1. Which of the following
pairs of bonds is the weakest? a. Cl and Cl b. H and H c. Br and Br d. F and F
2. Which of the following
has a positive entropy change? a. formation of
a liquid from a gas b. formation of
a solid from a gas c. formation of
a solid from a liquid d. 4 SO3(g)---->
4 SO2(g) + 2 O2(g)
3. Which of the following
single bonds is most reactive? a. H and H b. Br and Br c. F and F d. C and C
4. Which of the following
equilibrium constants has the biggest relative amount of products? a. K = 1 b. K = 0.0005 c. K = 2,000 d. K = 0
5. What is true for
a spontaneous reaction? a. enthalpy change
is always + positive b. free energy change
is always + positive c. entropy change
is is always - negative d. free energy change
is always - negative
6. Endothermic reactions
can never be spontaneous a. true b. false
7. An equilibrium constant
changes when the concentration of reactants is raised. a. true b. false
8. Which of the following
reactions is irreversible? a. 2 SO3
(g) <----> 2 SO2 (g) + O2 (g) b. 2 O3
(g) <----> 3 O2 (g) c. MgCO3(s)
<----> CO2 (g) + MgO (s) d. 2 C6H6(l)
+ 15 O2(g) <----> 12 CO2 (g) + 6 H2O
(g)
9. Which of the following
has an equilibrium expression with a only a "1" in the denominator? a. 2 O3
(g) <----> 3 O2 (g) b. MgCO3(s)
<----> CO2 (g) + MgO (s) c. C (s) + 2 Cl2
(g) <----> 2 CCl4 (g) d. 2 SO3
(g) <----> 2 SO2 (g) + O2 (g)
10. A catalyst alters
the rate of a reaction because a. it has weak bonds b. it weakens a
bond in reactants and changes the activation energy for the reaction c. it lowers the
activation energy for the forward reaction but not the reverse reaction d. decreases the
value for the equilibrium constant
Dr. Walt Volland,