Online Introductory Chemistry
Self Test on rates of reaction, equilibrium expressions, equilibrium constants, Le Chatelier's principle
Short essay questions KEY
1. What is a reversible reaction? A reversible reaction really involves two reactions. There is a "forward" reaction and a mirror image "reverse" reaction.The reactants combine to form products. The products "decompose" to form reactants. 2. Sketch the plot of reaction rate versus extent of reaction for reactants and products in a reversible reaction. 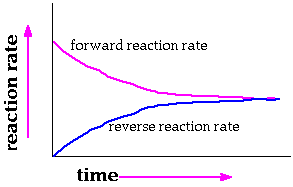
Describe how the rate for the forward reaction changes with time.The rate for the forward reaction starts out high because the concentration of reactants is highest at the start. The reaction rate decreases as time passes because the availability of reactants decreases.3. Write the equilibrium expressions for the following equations. Explain 2 SO3 (g) <----> 2 SO2 (g) + O2 (g) The equilibrium expression is also known as the mass action expression (MAE). It always is expressed in terms of products divided by reactants. The exponents on the concentrations come from the coefficients in the balanced equation. Dissolved substances and gases appear in the expression. Pure solids and liquids have an "activity" of "1" and are not included in the expression. Describe how the rate for the reverse reaction changes with time.The rate for the reverse reaction starts out at zero because the concentration of products that can be used in the reverse reaction is zero at the start. The reaction rate increases as time passes because the availability of products increases. Eventually the forward and reverse reaction rates are equal when equilibrium is reached. 4. Which of the following reactions has a positive entropy change and which has a negative one? Justify your answer. This reaction produces an increase in the number of gas molecules from 2 molecules of SO3 to three molecules, two SO2 and one O2 . This increases the disorder, entropy. The disorder for a system increases when the number of gas molecules increases. mass action expression or equilibrium expression = [ CO2 ]1 Pure solids are not included in the MAE. The concentration of "pure" solids and liquids does not change C (s) + 2 Cl2 (g) <----> 2 CCl4 (g)MAE = mass action expression = [ Cl2 ]2 / [CCl4 ]2 2 O3 (g) <----> 3 O2 (g) MAE = mass action expression = [ O2 ]3 / [O3 ]2 5. A reaction has a DH = +5600 calories. The entropy change is -4.6 calories /Kelvin at 298Kelvin. Is this reaction spontaneous? Will raising the temperature ever make it spontaneous?With the information provided, the Gibb's free energy must be calculated to decide if the reaction is spontaneous. The formula relating heat of reaction, enthalpy of reaction, and entropy is; DGo = +5600 calories - (298 Kelvin )(-4.6 calories /Kelvin) DGo = +5600 calories - (-1371 calories) D Go = +5600 calories - (-1371 calories) = +5600 calories +1371 calories) DGo = +6971 calories This amount of energy must be added to reactants to make the reaction occur. The positive sign for the free energy indicates the reaction is not self sustaining. It is not spontaneous. Raising the temperature will never make the reaction spontaneous. The enthalpy term is positive. The -TDSo term will always be PLUS no matter how high the temperature goes. This reaction produces an increase in the number of gas molecules from zero gas molecules to one. This increases the disorder. The disorder for a system increases when the number of gas molecules increases. This reaction produces no increase in the number of gas molecules. There are two gas molecules in reactants and two gas molecules in products. The disorder for the system remains essentially constant when the number of gas molecules stays the same. This reaction produces an increase in the number of gas molecules from 2 molecules to three. This increases the disorder. The disorder for a system increases when the number of gas molecules increases. 6. An equilibrium reaction has the following equilibrium expression. Assume DH = -75,600 calories. What is the effect on [SO2 ] if the pressure is increased? If the pressure is increased the reaction will re-establish equilibrium by decreasing the amount of [O2] and [SO2 ] . The additional [SO2 ] will be consumed to create an increase in the amount of reactant, [SO3]. The reaction will shift to form more reactant, [SO3]. What happens to [SO2 ] if the temperature is raised? The reaction is exothermic. The minus sign for the enthalpy change tells that. An increase in temperature for an exothermic reaction shifts the equilibrium towards the reactants. Raising the temperature of an exothermic reaction "favors" reactants. 7.Give definitions for activation energy, heat of reaction, endothermic, exothermic, spontaneous reaction. What is the standard used for predicting spontaneous reactions?Check the lessons at the following URLsEffects of temperature, concentration, catalysts, inhibitors on reaction ratesandFree Energy The Reason Why a Reaction OccursandExothermic and Endothermic Reactions, Calculating Energy ChangesWhat effect does temperature have on reaction rates? Reaction rates always increase when the temperature is raised. This happens because increased temperatures increase the number of collisions between reacting particles. Increasing the temperature also increases the energy and force of collisions making more of them "effective" in breaking up reactants and forming products. Can an activation energy ever be negative? Activation energies are always positive or zero. The activation energy is the amount of energy needed to "initiate" a reaction by weakening bonds in reactants and forming a temporary particle called an activated complex or transiion state. Explain your answer. .
1.Which of the following pairs of bonds is the weakest? a. Cl and Cl b. H and H c. Br and Br d. F and F
2.Which of the following has a positive entropy change? a. formation of a liquid from a gas b. formation of a solid from a gas c. formation of a solid from a liquid d. 4 SO3(g)----> 4 SO2(g) + 2 O2(g) when the number of gas molecules increases the entropy increases for the system
a. H and H b. Br and Br c. F and F the weaker the bond the more reactive it is d. C and C
a. K = 1 b. K = 0.0005 c. K = 2,000 The largest equilibrium constant has the largest relative amount of product d. K = 0
a. enthalpy change is always + positive b. free energy change is always + positive c. entropy change is is always - negative d. free energy change is always - negative Whenever the free energy change is negative the process is spontaneous. The process will happen of its own accord one it is started.
a. true b. false A reaction can be spontaneous if the DH is positive as long as the entropy change is positive . This means the free energy change will be negative because DG = DH - TDS
a. true b. false
a. 2 SO3 (g) <----> 2 SO2 (g) + O2 (g) b. 2 O3 (g) <----> 3 O2 (g) c. MgCO3(s) <----> CO2 (g) + MgO (s) d. 2 C6H6(l) + 15 O2(g) <----> 12 CO2 (g) + 6 H2O (g)
a. 2 O3 (g) <----> 3 O2 (g) b. MgCO3(s) <----> CO2 (g) + MgO (s) c. C (s) + 2 Cl2 (g) <----> 2 CCl4 (g) d. 2 SO3 (g) <----> 2 SO2 (g) + O2 (g) 10.A catalyst alters the rate of a reaction because a. it has weak bonds b. it weakens a bond in reactants and changes the activation energy for the reaction c. it lowers the activation energy for the forward reaction but not the reverse reaction d. decreases the value for the equilibrium constant |