|
|
|
Collision theory and temperature effects on rates |
|
Kinetic theory says that molecules are in constant motion.The velocity of the molecules is directly proportional to the Kelvin temperature. The kinetic energy and molecule velocity increase with temperature. KE = [1/2][mv2] The mass of the molecule is "m" and the velocity is "v". visit for kinetic energy interactive exercise .
|
|
New bonds can form only if the atoms are close enough together to share electrons. The collisions need to be violent (energetic) enough to break bonds in the reactant molecules. Some collisions are not successful. These are called ineffective collisions. The molecules simply hit and then bounce off one another. This animation illustrates what happens in an ineffective collision. Reactions usually require effective collisions between reactant molecules or atoms. The formation of bonds requires atoms to come close to one another. |
|
|
Collisions that lead to products are called effective collisions. An effective collision must happen with a great enough speed, energy and force to break bonds in the colliding molecules. The animation illustrates an effective collision between two diatomic molecules. The two product molecules formed fly outwards.
|
|
|
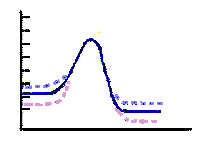
Temperature and molecule velocity Collisions between molecules will be more violent at higher temperatures. The higher temperatures mean higher molecular velocities. This means there will be less time between collisions. The frequency of collisions will increase. The increased number of collisions and the greater violence of collisions results in more effective collisions. The rate for the reaction increases. Reaction rates are roughly doubled when the temperature increases by 10 degrees Kelvin. This means the rate can be quadrupled if the temperature is raised by 20 degrees Kelvin. site with interactive temperature effect on velocity. It makes sense that if we can increase reaction rates by increasing temperature you can decrease reaction rates by lowering the temperature. We do this every time we put something in the refrigerator. We can see the effect of elevated temperatures and increased reaction rates at home. If we leave a dairy product out of the refrigerator for a few days it will spoil. A frozen item of the same age dairy product will still be useable. Temperature effects on rates and activation energy diagram
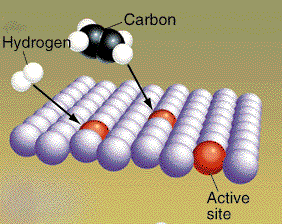
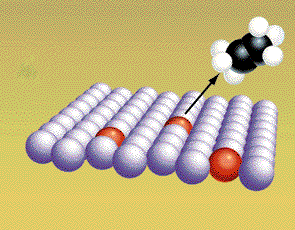
Concentration Reaction rates can be increased if the concentration of reactants is raised. An increase in concentration produces more collisions. The chances of an effective collision goes up with the increase in concentration. The exact relationship between reaction rate and concentration depends on the reaction "mechanism". This is the process involving elementary reaction steps. The slowest step controls the rate. The nature of the slow step is not obvious from the balanced equation. Only experimental observation reveals the link between concentration and reaction rates. Catalysts and inhibitors The reaction energy path controls the speed of the reaction. The molecules follow the path of least resistance, but this path may still require a lot of energy. The activation energy for the path may be high and then the reaction will be slow. visit page for general information on catalysis visit for catalytic converter information visit for gold interactive uses as catalyst etc A reaction pathway can be altered by adding nonreacting compounds to the reaction mixture. These molecules can sometimes alter the pathway so the energy needed for reaction is lowered. When this happens the reaction rates are faster. A material that lowers the activation energy is called a catalyst. The four pictures show the effect of a catalyst on hydrogenation of ethylene. The CH2CH2 and H2 molecules are attracted to the atoms of the metal catalyst. (Graphic by permission of Prentice Hall)
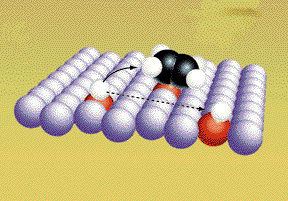
The covalent bonds are weakened because the metal atoms attract electrons away form the "bonded" atoms. The bonds between the hydrogen atoms are weaken more and break. (Graphic by permission of Prentice Hall)
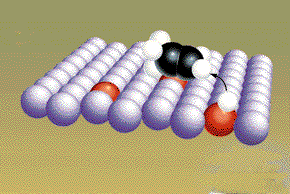
The free radicals from the original hydrogen molecules move along the surface of the metal until they collide and form products. (Graphic by permission of Prentice Hall)
|