|
Reaction Rates & Why Reactions Take Time |
|
Activation energy diagrams: going uphill or Starting a reaction |
|
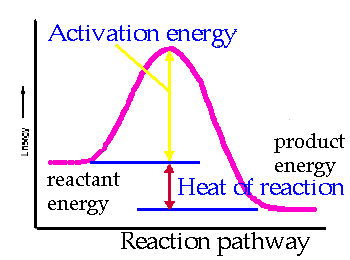
The reaction energy path controls the speed of the reaction. The molecules follow the path of least resistance, but this path may still require a lot of energy. A picture of the energy of reactants on their way to form products is an energy diagram. The energy requirement is called the activation energy. The activation energy is always positive. for the path may be high and then the reaction will be slow. A common analogy is pushing a boulder over a hill. Actually over a "pass" . The reactants are on one side like the boulder. The energy needed to push the boulder to the crest of the hill is like the activation energy. The products are like the condition when the boulder is at the bottom of the far side of the "pass". |
|
|
The ground elevations are all tied to the energies of the reactants, activated complex, and products. The vertical axis of the plot is potential energy. The x axis or horizontal axis is the extent of reaction or reaction path. The illustration shows the activation energy marked with a pale yellow arrow. It is the difference between the energy of the activated complex at the top of the hill and the energy of reactants. The red arrow marks the heat of reaction. This is the difference in energy between the products and the reactants. The picture shows an exothermic reaction. |
|
|
|
|
Exothermic reactions, endothermic reactions, and effect of catalysts |
|
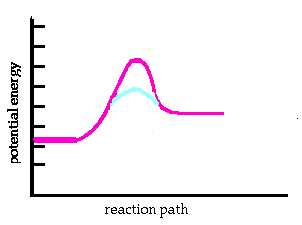
An activation energy diagram for an endothermic reaction is illustrated here. The potential energy for products is higher than the energy for reactants. The magenta curve is the reaction pathway for an uncatalyzed reaction. The cyan (blue) curve shows what a catalyst does to the energy for the reaction path. The reaction is faster when the catalyst is present. The activation energy is lower with the catalyst present. NOTE: The energies of reactants and products have not changed. The heat of reaction is the same. The relative amounts of reactants and products stay the same. The catalyst only allows the reaction to reach equilibrium faster. |
|
|
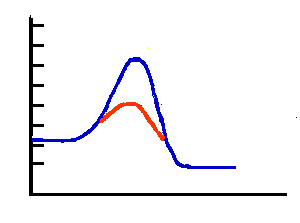
In this illustration the blue curve shows the reaction energy diagram for an exothermic reaction. The products have lower potential energy than the reactants. The red curve shows what a catalyst does to the activation energy and reaction path. The reaction has the same heat of reaction but a much lower activation energy. The catalyzed reaction would be much faster. NOTE: The energies of reactants and products have not changed. The heat of reaction is the same. The relative amounts of reactants and products stay the same. The catalyst only allows the reaction to reach equilibrium faster. |
|
|
Examples: |
|
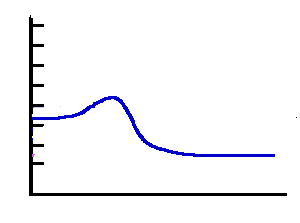
Exothermic 1 |
|
|
|
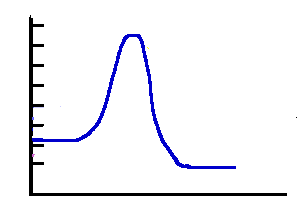
Exothermic 2 |
|
|
|
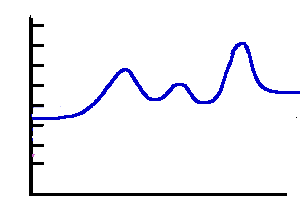
Endothermic 3 |
|
|
|
Exercise. |
|
Which of the previous three reactions has the smallest activation energy? Which one is the fastest reaction? |
|
Answer: The"Exothermic 1" has the lowest activation energy and the fastest rate. |
|
Online Introductory Chemistry |
|
|
|
|