|
Reversible
Reactions and Chemical Equilibrium
|
|
Reversible
Reactions and Chemical Equilibrium
|
|
When reactions are
analyzed in kinetics, there are two types of reactions. There are nonreversible
reactions and reversible reactions.
|
|
The nonreversible
reactions occur so that the reactants are completely consumed in the
reaction, the products are formed and the process comes to an end. These
kinds of reactions are said to go to "completion". Burning a piece of
paper or metabolizing glucose, C6H12O6(s),
is a nonreversible reaction. Once the oxygen and paper form CO2
and H2O the process is over.
|
C6H12O6(s)
+ 6 O2 (g) ------> 6 H2O(g) + 6 CO2
|
Some ambitious plant
or bacterium somewhere may take in the water and carbon dioxide to make
cellulose again, but that doesn't fit the idea of a reversible reaction.
|
|
A
reversible
reaction is an interesting process. The reaction really involves two
reactions. There is a "forward" reaction and a mirror image "reverse"
reaction.
|
H2(g)
+ F2(g) ------> 2 HF(g) forward
|
|
H2(g)
+ F2(g) <------ 2 HF(g) reverse
|
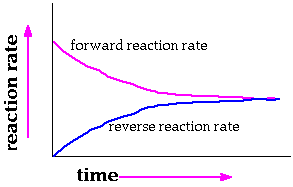
Reversible chemical
reactions follow a similar pattern. The reactants are initially the
only molecules around. They react to form products. The amount of reactants
dwindles and the forward reaction slows down. The product amounts increase
at the same time the reactants are disappearing. They products "decompose"
to form reactants. The rate for this reverse reaction increases as the
amount of product grows. Ultimately there comes a time when the forward
reaction rate and the reverse reaction rates are equal. The mixture
is at equilibrium.
At equilibrium the
numbers of reactant and product molecules stays constant. The identity
of individual molecules keeps changing.
|
|
|
|
Dr.
Walt Volland, , All rights reserved, 1998
|
|
last
modified April 22, 2004
|