|
|
|
Henry's Law |
|
The solubility of a gas in a liquid depends on temperature, the partial pressure of the gas over the liquid, the nature of the solvent and the nature of the gas. The most common solvent is water. Carbonated beverages are an example of Henry's law in everyday life. The dissolved carbon dioxide stays in solution in a closed pop bottle or can where the partial pressure of carbon dioxide was set at a high value during bottling. When the can or bottle is opened the partial pressure of CO2 is much lower and the dissolved carbon dioxide will gradually escape from the pop. When the new low partial pressure equilibrium is established the soda will be "flat" . This loss of dissolved carbon dioxide will happen faster for warm soda than for cold. Gas solubility is always limited by the equilibrium between the gas and a saturated solution of the gas. The dissolved gas will always follow Henry's law. The concentration of dissolved gas depends on the partial pressure of the gas. The partial pressure controls the number of gas molecule collisions with the surface of the solution. If the partial pressure is doubled the number of collisions with the surface will double. The increased number of collisions produce more dissolved gas. The illustration shows that if the pressure is doubled then the concentration of dissolved gas will double.
|
|||||||||||
|
Low pressure
equilibrium Low
concentration Double the pressure
equilibrium Double the
concentration
The dissolving process for gases is an equilibrium. The solubility of a gas depends directly on the gas partial pressure. At equilibrium the number of molecules leaving the gas phase to enter the solution equals the number of gas molecules leaving the solution. Gas solubility is proportional to the gas partial pressure. If the temperature stays constant increasing the pressure will increase the amount of dissolved gas.

The Henry's law constant "k" is different for every gas, temperature and solvent. The units on "k" depend on the units used for concentration and pressure. The value for k is the same for the same temperature, gas and solvent. This means the concentration to pressure ratio is the same when pressures change. The following equation can be used to relate pressure and concentration changes for solutions at the same temperature. The initial condition has concentration C1 and gas partial pressure P1. The second condition has concentration C2 and gas partial pressure P2.
|
|
Example: |
|
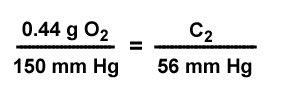
What is the predicted concentration of dissolved oxygen, if the partial pressure for oxygen is 56 mm Hg? The concentration of dissolved oxygen is 0.44 g / 100 ml solution. The partial pressure of oxygen is 150 mm Hg. |
|
Solution: |
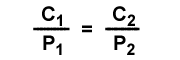
|
|
P1 = 150 mm Hg--------------------------------------------- |
C1 = 0.44 g O2 /100 ml solution |
|
P2 = 56 mm Hg----------------------------------------------- |
C2 = ? |
|

|
|
|
Online Introductory Chemistry Dr. Walt Volland all rights reserved revised November 12, 2011