


|
Online
Introductory Chemistry
Solubility: How solubility is
measured
|
|
The amount of solute that
can be dissolved in a definite amount of solvent is used as
the measure of solubility. The conventional reference for
solubility is the number of grams of solute that can
dissolve in 100 mL of solvent. Sometimes the solubility is
in grams of solute per 100 grams of solvent. The table below
gives typical solubility data for some common inorganic
compounds.
|
|
Solubility of Common
inorganic compounds in grams solute per 100 mL of
water
|
|
Substance
|
0oC
|
10oC
|
20oC
|
30oC
|
40oC
|
50oC
|
|
KI, potassium
iodide
|
127.5
|
136
|
144
|
152
|
160
|
168
|
|
KCl, potassium
chloride
|
27.6
|
31.0
|
34.0
|
37.0
|
40.0
|
42.6
|
|
NaCl, sodium
chloride
|
35.7
|
35.8
|
36.0
|
36.3
|
36.6
|
37.0
|
|
NaHCO3 , sodium
bicarbonate
|
6.9
|
8.15
|
9.6
|
11.1
|
12.7
|
14.45
|
|
NaOH, sodium
hydroxide
|
-----------
|
-------------
|
109
|
119
|
145
|
174
|
|
MgSO4• 7
H2O, epsom salts
magnesium sulfate
heptahydrate
|
------------
|
23.6
|
26.2
|
29
|
31.3
|
-------------
|
|
|
|
|
|
|
|
|
|
These values are the amount
of solute that will dissolve and form a saturated solution
at the temperature listed. A saturated solution is one where
there is an equilibrium between undissolved solute and
dissolved solute.
|
NaCl(s)
<---> Na1+(aq) +
Cl1-(aq)
|
The solvent cannot dissolve
more solvent at that temperature. The solubility can be
increased if the temperature is increased. The table shows
that solubility usually increases with increasing
temperature. Clearly there are exceptions such as
Ce2(SO4)3
|
|
Solubility of common
inorganic compounds in grams solute per 100
of solvent
|
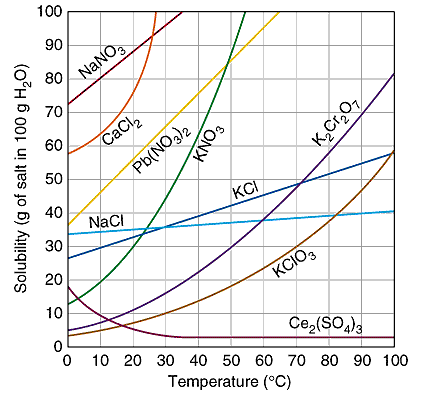
|
Reading the Solubility
Plot
|
|
A saturated KCl solution at
10oC will have 31 grams of KCl dissolved in 100
grams of water. If there are 40 grams of KCl are in the
container, then there will be 9 grams of undissolved KCl
remaining in the solid.
Raising the temperature of
the mixture to 30oC will increase the amount of
dissolved KCl to 37 grams and there will be only 3 grams of
solid undissolved. The entire 40 grams can be dissolved if
the temperature is raised above 40oC.
Cooling the hot
40oC solution will reverse the process. When the
temperature decreased to 20oC the solubility will
eventually be decreased to 34 gram KCl. There is a time
delay before the extra 6 grams of dissolved KCl
crystallizes. This solution is "supersaturated" and is a
temporary condition. The "extra" solute will come out of
solution when the randomly moving solute particles can form
the crystal pattern of the solid. A "seed" crystal is
sometimes needed to provide the surface for solute particles
to crystallize on and establish equilibrium.
|
|
Online Introductory
Chemistry
|
Dr. Walt Volland
all rights reserved revised March 29, 2005
