|
How Things Dissolve Dr. Walt Volland all rights reserved, 1997-2011 revised November 11, 2011 |
|
Solutions |
|
We make solutions all the time. We use them when we make instant coffee, wash clothes, add water to fruit juice concentrates, and on and on. The Workings of our body depend on transfer of matter in solution and suspensions. We discussed the mechanical solution process when we studied mixtures. Here are some terms you need to know. Solvent : This is the substance in a solution that is present in the greater amount. It is the major part of a solution and determines the physical properties o f the mixture. Solute: This is a substance that is present in the lesser amount. It is the smaller portion of a solution. Solution: A solution is a homogeneous physical mixture of solvent and solute. The solute particle sizes are ions, atoms, molecules or small combinations of these units. |
|
Lets review these ideas by looking at what happens when sugar dissolves in water. We know water molecules are bent. The oxygen end of the vee shaped molecule has a slight negative charge, d-. The two hydrogen ends of the vee have a slight positive charge, d+.
|

|
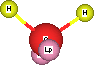 
|
|
Space filling water model showing lone pairs in "pink" |
Ball & stick model of water showing lone pairs in "pink". |
|
The glucose sugar molecule, C6H12O6, is an organic molecule. It was classified this way because glucose is made by living organisms. This makes it "organic". Glucose has six -O-H groups along the carbon skeleton. These -O-H are polar centers. The symmetry of glucose decreases the polarity of the molecule, but the "O" in each -O-H has a has a slight negative charge, d-. The hydrogen end of the -O-H has a slight positive charge, d+. Remember there are "6" of these in glucose.
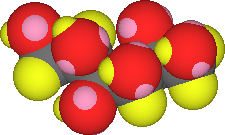
When the attractive forces of the water molecules for the glucose exceeds the attractive forces between the glucose and its neighboring glucose molecules the water can rip the sugar molecule out of the crystal. The glucose is "solvated" when it surrounded solvent molecules. The solvent has "dissolved" the molecule. This process is repeated over and over again until either the sugar is completely dissolved or the supply of unattached water molecules is exhausted. |
|
There is a definite number of water molecules needed to solvate or isolate a glucose solute molecule. Once the solvent population is all tied up, no more sugar (solute) can dissolve. You can see this kind of thing happen if you add table sugar (sucrose, C12H22O11) to water. At first the solid disappears, but eventually additional solid sinks to the bottom. The solution is saturated. |
|
Miscible and miscibility: |
|
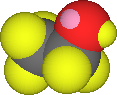
This limitation on the solubility of a solute doesn't apply if the solute and solvent are very similar. For example water, H2O, and ethyl alcohol, CH3CH2OH are completely "miscible". Both water and ethanol are polar molecules with hydrogen bonding between molecules too. The great similarity of the two molecules results in solutions where the water molecules and CH3CH2OH are interchangeable. The molecules are in sense "dumb" and can't distinguish one from the other. There is no limit to the possible concentration of water in ethanol or ethanol in water. |
|
|
|
|
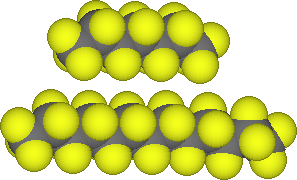
Organic compounds like n-hexane, C6H14, and dodecane, C12H26, are miscible in one another. The nonpolar molecules are attracted to one another through London forces (dispersion forces). The molecules in solution are essentially interchangeable. These examples illustrate what is meant by the comment "like dissolves like" . Polar compounds dissolve in polar compounds and nonpolar dissolve in nonpolar. The types of intermolecular forces dictate these results.
|
Online Introductory Chemistry Dr. Walt Volland all rights reserved, 1997-2011 revised November 11, 2011