Online
Introductory Chemistry
Dilution
Online
Introductory Chemistry
Dilution
|
Dilution |
|
Concentrated solutions can be mixed with solvent to make weaker or dilute solutions. This is the kind of thing people do everyday with consumer products like fruit juice. Some concentrated solutions are used as "stock" solutions. Weaker solutions are typically used but the concentrated solutions require less storage space. In recent years accidents have occurred in the health care professions when dilutions were done incorrectly. Some of these errors have resulted in deaths or serious injuries. A number of health care facilities have abandoned the practice of "diluting" stock solutions because dilution instructions were too hard to follow. They do not want to take the risks associated with errors in preparing diluted solutions. |
|
Moles of solute in a known volume of solution: |
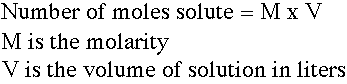
This relation is used to determine the number of moles of solute in a known volume of solution. The volume is converted to liters. There are some people who use "millimoles" and leave volumes in milliliters. The unit labels have to be used carefully. |
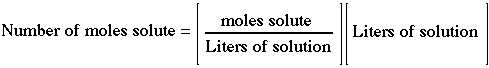
|
Example: |
|
How many moles of glucose are in 275 mL of 4.0 M glucose solution?? |
|
Step 1 |
Convert the volume from milliliters to liters.
|
|
Step 2 |
Use the definition of molarity to calculate the moles of solute.
|
|
Molarity and dilutions |
|
In dilutions the amount of solvent is increased, but the amount of solute is kept constant. The result is a decreased concentration, but a greater volume. The idea is that the volumes may change but the number of moles does not. This means that the original number of moles and the final number of moles are the same. number moles original = number of moles final Moriginal x Voriginal = Mfinal x Vfinal |
|
The word "numbers of moles" can be replaced by the molarity, M, and volume,V. |
|
This equation can be used to figure out any one of the terms if given the other three. What is also true is that the difference between the original volume and the final volume tells how much solvent is needed to make the desired "dilution" Vadded = Vfinal - Vinitial |
|
You have some "checks" to prevent errors. The first is that the final volume always has to be more than the original volume. The second is that the final molarity is always less than the original. |
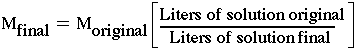
The ratio of the original volume to the final volume is sometimes called the dilution factor. It is always a number smaller than "1". That is why the concentration at the end is less. |
|
Example: Final molarity after dilution |
|
What is the final molarity of a solution made when 380. mL of 0.48 M NaCl solution is diluted to a final volume of 800. mL? |
|
Step 1 |
Convert the volumes from milliliters to liters.
|
|
Step 2 |
Use the definition of molarity to calculate the moles of solute.
|
|
Sometimes people need to know how much original solution is needed to make a specific volume of a dilute solution. |
|
Example: Initial volume needed to make a definite molarity after dilution |
|
What original volume of 3.00 M HCl solution is needed to make 62.0 mL of 2.00 M HCl solution? How much water was added? |
|
Step 1 |
Convert the final volume from milliliters to liters.
|
|
Step 2 |
Identify original and final quantities.
|
Step 3 |
Use the dilution equation to calculate the value for the unknown original volume. volume original = Voriginal = Vfinal [ Mfinal / Moriginal ] volume original = Voriginal = [0.0620 L solution ] [ 2.00 M HCl / 3.00 M HCl ] volume original = 0.0400 L Three significant figures volume of water added = 62.0 ml - 40.0 ml = 22.0 ml |
Dr. Walt Volland all rights reserved 1997-2005 revised March 29, 2005
| |