Electrolytes, nonelectrolytes and percent ionization
Electrolytes, nonelectrolytes and percent ionization
|
Electrolytes |
|
Electrolytes are substances that form solutions that conduct electricity. Pure water does not conduct electricity. Water molecules do not dissociate significantly to form charge carriers (ions). |
|
Electrolytes dissolve in water or some other solvent and form ions. Sodium chloride is an electrolyte. When NaCl dissolves in water the crystal is separated into Na1+ and Cl1- ions. The ions are surrounded by solvent molecules. The clumps of solvent molecules and ions are mobile in the solution. The clumps are centerd on the charged ions and are able to carry electrical charge through the liquid. |
|
Strong electrolytes are substances that convert completely to ions when they dissolve. They are also said to be 100% ionized. The solubility of ionic compounds limits their ability to conduct. |
|
Some strong electrolytes are covalent compounds like HNO3 or HCl. These compounds also ionize 100% in aqueous solutions. 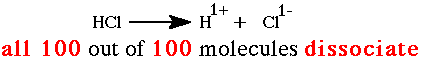 |
|
Weak electrolytes dissolve and only a small fraction of the formula units dissociate to form ions. Solutions of weak electrolytes are poor conductors of electricity. Acetic acid is a weak elecetrolyte. |
|
When 100 molecules of acetic acid dislove in water 96 of the molecules remain intact and 4 of the molecules ionize. This means that the acetic acid is only 4% ionized or dissociated. 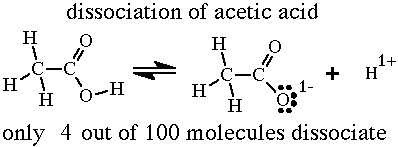 The hydrogen in the -O-H group is acidic. The two oxygens bonded to the same carbon atom attract electrons formthe carbon and neighboring atoms. This draws electrons from the O-H bond. This makes the O-H bond weaker than normal. But the O-H bond is still strong enough to remain intact 96 % of the time. |
|
Nonelectrolytes dissolve and all of the formula units remain intact and none of them dissociate to form ions. Solutions of weak electrolytes are poor conductors of electricity. Ethyl alcohol and methyl alcohol are nonelectrolytes. None of the CH3OH or CH3CH2OH molecules dissociate and form ions. A solution of hexane and dodecane will not conduct electricity because there are no ions formed by the solute and solvent. |
|
Online Introductory Chemistry |
Dr. Walt Volland all rights reserved 1997-2005 revised March 29, 2005