

Arrhenius acid
definition
- Definition of
an Arrhenius acid
- Acids
are compounds that can donate H+ ions in
water
solutions.
The role of water is essential in this definition. Pure
HF is a gas and is not considered to be an acid. It must
be dissolved in water to act like an acid. There are many
different acids, but they have something in common. They
all have hydrogen attached to a nonmetal from either
group 6A or group 7A. This last idea makes it easier to
identify acids.
-
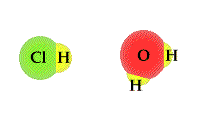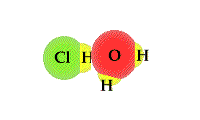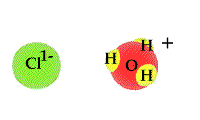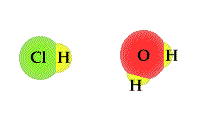
- The
process in which water accepts a proton from an acid can
be viewed as a TUG of WAR between the acid and
water. An acid will release a proton to a water molecule.
The proton will be covalently bonded to the "O" in water
through a covalent bond. The unshared electron pair on
the "O" has a strong attract for the proton than the
Cl1- ion hydrochloric acid.
-
 -
-
- Binary
acids are acids that have hydrogen combined with an
atom from either group 7A or 6A. The examples of binary
acids are:
-
- HF,
HCl, HBr, HI, H2S and H2O.
- It may
be difficult to picture water as an acid but under
certain conditions the water molecule breaks up to form
H+ ions and OH- ions. This is one
of nature's tricks. Pure water is not typically
considered very acidic, because an OH- ions is
released along with every H+ ion.
-
- A
hydrogen must be covalently bonded to O, S, F, Cl, Br, or
I in order to be acidic. This requirement must be met.
Any other combination of H and nonmetal element will
generally not be weak enough to be broken and form
protons in water.
-
|

