- Definition
of an oxacid
- Acids are
compounds that can donate H+ ions in
water solutions.
-
- Ternary acids
also known as OXOACIDS or oxyacids contain three
elements, H , O and a central atom, like HNO3. These types of
acids usually have hydrogen attached to oxygen. The
hydrogens are on the "edge" of the moleculare
structure. The oxygen is conected to a central atom.
Examples of common ternary acids are:
-
-
HNO3,
HNO2, H2SO4, H2
SO3, H3PO4,
H3PO3,
H2CO3
-
- You should see
a common characteristic in these acids. They all have
a nonmetal element as a central atom. They all have
oxygens that are bridges between the hydrogen and the
central atom. These substances are acids because the connection or bond between H and O is weak in
these compounds. The H-O bond breaks with the formation of
a H+ ion and a polyatomic anion.
-

-
|
nitric acid
|
|
water
|
|
proton
|
|
nitrate ion
|
HNO3 |
|
+ H2O |
----> |
H1+ (aq) |
|
+ NO31- (aq) |
HNO3+
H2O
---> H1+ (aq) +
NO31- (aq)
-
The
(aq) means the ion is dissolved in water. The ion is
in an aqueous mixture.
- The oxy acids
or ternary acids have their origins in oxides of the
nonmetals like CO2(gas). The strange thing
about this is that these nonmetal oxides form acids
when they mix with water. These compounds are the
villains that cause the acid rain problem.
-
-
|
carbon
dioxide
|
|
water
|
|
carbonic
acid
|
|
CO2
|
+ H2O
|
---> H2CO3
(aq)
|
The (aq) means the particle is dissolved in
water. The particle is in an aqueous mixture.
- Polyprotic
acids
- Acids with more
than one acidic proton/hydrogen can release all of the
protons. Thedescription of these acids ilinked to the
multiple number of acidic hydrogen atoms. A diprotoic
acid has "two" acidic hydrogens. A triprotic acid has
"three" acidic hydrogens. This means
H2SO4 has two acidic hydrogens
that can be released form each formula
unit.
-
|
sulfuric
acid
|
|
water
|
|
two protons
|
|
sulfate ion
|
|
H2SO4
|
+
|
H2O
|
------>
|
2
H+ (aq)
|
+
|
SO42-(aq)
|
The (aq) means the particle is dissolved in
water. The particle is in an aqueous
mixture.
-
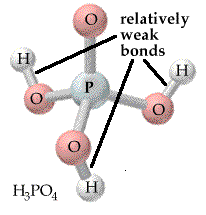
- A triprotic
acid is phosphoric acid,
H3PO4
-
-
- Non-acidic
hydrogens
- These ideas
explain why hydrocarbon compounds like gasoline and butane, C4H10, are
not
acids even
though there are many hydrogen atoms in the molecule.
In hydrocarbons, hydrogen atoms are attached to carbon. The C-H bond is
nonpolar and strong. Hydrocarbons are not acidic because H is not bonded to an atom in
Group 6A or 7A.
-
-
CH3CH2CH2CH3.
-
-
A
key idea is that an acidic hydrogen atom must be bonded
-
F,
Cl, Br, I, O or S
-
|