
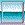

Arrhenius Bases
- Arrhenius
Definition of a base
- Arrhenius bases
are compounds that donate OH- ions
in water solutions.
The role of water is essential in this definition. Pure
solids like NaOH must be dissolved in water to act like a
base. There are many different bases, but they have
something in common. They all have an hydroxide ion,
OH1- and a metal. This last idea makes it
easier to identify bases.
-
- Bases have a
hydroxide ion combined with a metal. The examples of
bases are:
-
- LiOH, KOH,
NaOH, CsOH, RbOH and H2O.
- Mg(OH)2,
Ca(OH)2, Ba(OH)2,
Sr(OH)2
- Al(OH)3,
Sn(OH)2,
Pb(OH)2,
Fe(OH)2,
Fe(OH)3
-
- You should see a
common characteristic in these bases. They all have a
metal element and a hydroxide ion. The reason these
substances are bases is that they dissolve in water to
release hydroxide ions. The limitation on these compounds
is their solubility in water. Typically all metal
hydroxides are bases. The problem for these compounds is
that only group 1 and 2 are very soluble in water.
|
magnesium
hydroxide
|
|
water
|
|
magnesium ion
|
|
hydroxide ion
|
|
Mg(OH)2
|
+
|
H2O
|
---->
|
Mg
2+
(aq)
|
+
|
2
OH1- (aq)
|
The (aq) means the ion is dissolved in water. The
ions are in an aqueous mixture. The water does not get
balanced
|