Dissociation, self-ionization of water, Kw
Pure water is not really pure. The purest water contains some hydronium ions and hydroxide ions. These two are formed by the self-ionization of two water molecules. This happens rarely. The process is an equilibrium where the reactants, intact water molecules, dominate the mixture. At equilibrium the molarities for the hydronium ion and hydroxide ion are equal. [H3O1+] = [OH1-] The animation shows one of the effective collisions between two water molecules to form hydroxide and hydronium ions. The process is reversible. Water normally exists as a mixture of molecules, hydroxide ions and hydronium ions. The mixture is dominated by water molecules with only traces of the ions. 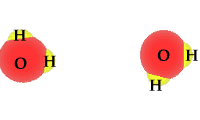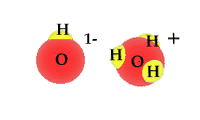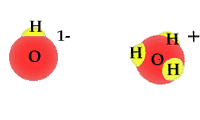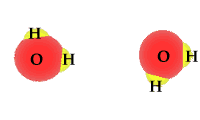
The equation is H2O + H2O <---> H3O1+ + OH1- The equilibrium expression is the normal products over reactants. K = [H3O1+] [OH1-] / [H2O] [H2O] The molarity for the water is a constant at any specific temperature. This means the equation can be rewritten as K[H2O] [H2O] = [H3O1+] [OH1-] The quantity on the right hand side of the equation " K[H2O] [H2O] = Kw " is formally defined as Kw. The numerical value for Kw is different at different temperatures. At 25oC Kw = 1.0 x 10-14 Kw = K[H2O] [H2O] Kw = [H3O1+] [OH1-] = 1.0 x 10-14 |