|
Calculate the mols of carbon dioxide
% yield = 100 x ( # moles carbon dioxide actual / # moles of carbon dioxide expected) alternately in terms of yield weights |
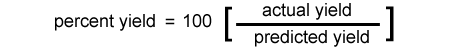
|
your instructor. Paste the report in an email.
For people in Canvas you will submit an equiz inside Canvas for your report.
Chemical Reactions Report Sheet
Data
|
|
|
|
|
|
|
Water used to fill plastic bottle: |
|
|
|
|
|
|
1) |
ml |
ml |
ml |
|
|
2) |
ml |
ml |
ml |
|
|
3) |
ml |
ml |
ml |
|
|
4) |
ml |
ml |
ml |
|
Total volume of plastic bottle |
ml |
ml |
ml |
|
|
Volume of water left in bottle after CO2 was collected |
ml |
ml |
ml |
|
|
Mass of baking soda used |
g |
g |
g |
|
Calculations
|
|
|
|
|
|
Moles of baking soda, NaHCO3 used |
moles |
moles |
moles |
|
Moles of CO2 that can be made from this many moles of NaHCO3 (theoretical yield of CO2) ** Show how |
moles |
moles |
moles |
|
Volume of CO2 trapped in bottle |
ml |
ml |
ml |
|
Volume of CO2 trapped in bottle |
L |
L |
L |
|
Moles of CO2 trapped in bottle (actual yield of CO2) |
moles |
moles |
moles |
|
% yield of CO2 |
% |
% |
% |
**Calculation set-up for finding calculated or theoretical yield of CO2 for Trial 1.
Percent yield average
|
|
Trial 1 |
Trial 2 |
Trial 3 |
Average Percent Yield |
|
%
yield of CO2 |
% |
% |
% |
% |