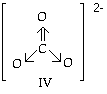
Predicting Molecular Shapes using models and VSEPR
|
|
||
| Triangular Planar | Trigonal Pyramid | Tetrahedral |
| Molecules with single bonds | Molecules with multiple bonds | Molecules with 2 central atoms |
|
AXE,
|
ABE, where X = B |
|
|
Dr. Walt Volland All rights revised© July 9, 2013
|
This exercise is aimed at using Lewis dot structures and building molecular models to predict the shapes for small molecules and fragments that are building blocks for large molecules like DNA, RNA, proteins, fats, and carbohydrates. The basic model used in this exercise is the Valence Shell Electron Pair Repulsion Theory. It is essential to "build" the models so you have a 3D experience with these shapes. Click to view a toothpick & pladoh model of a tetrahedral shape. Two-dimensional Lewis dot formulas help us understand the bonding within a molecule or polyatomic ion, but they do not give us a sense of the 3-dimensional shape of the particle. Valence Shell Electron Repulsion Theory (VSEPR) is often used to predict particle shape from a Lewis dot formula. The principle is that the electron pairs(groups) repel one another to achieve a minimum in repulsion energy between the electrons. Click here for in depth VSEPR content. The VSEPR theory focuses on the idea that electrons repel one another and that these repulsions are smallest when the electron pairs or groups of electron pairs are as far apart as possible. This will then be the most stable form or shape of a particle. Electron groups in the VSEPR model are lone pairs, single bonds, double bonds, triple bonds and single electrons as in NO. The No molecule is an exception to the octet rule. There are only 11 valence electrons in the molecule and the nitrogen cannot "get" an octet. The oxygen has a higher electronegativity and does form an octet. We know from a study of Lewis formulas that molecules and polyatomic ions may contain single bonds, double bonds, triple bonds, and "lone pairs" . The lone pairs of electrons not used for bonding. We also know that a structure contains one or more "central atoms" around which the rest of the atoms are arranged; we know that the rest of the atoms are bonded either directly or through other atoms to this center atom. Remember the central atoms in molecules usually have attained an octet of valence electrons. This is the reason the central atom is bonded. The central atom in the structure now has an octet around it, not just the original set of valence electrons. In the VSEPR approach molecule shapes, you focus on two things a central atom and electron groups around it .
Representations of the molecule use the symbol , AXnEm, or ABnEm where A = central atom, X = atoms joined to A by bonds, E = lone pairs on A. The subscript n equals the count of bonded atoms and the subscript m equals the count of unshared pairs. The sum of m and n tells the number of electron clouds or groups around the central atom. This helps us predict the shape around the central atom. |
|
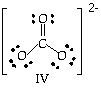
The arrangement in space (geometry ) of the electron groups around a center atom controls the overall shape because all bonds and unshared pairs radiate out from the central atom. An electron group may be 1 pair of electrons (single bond or lone pair), 2 pairs (double bond) or 3 pairs (triple bond). The carbonate ion, for example, has one double bond and two single bonds attached to the center carbon atom. Thus, there are 3 groups of electrons around the C even though there are 4 pairs (an octet) of electrons on carbon. Two pairs of electrons point in the same direction, the double bond to O. The other two pairs go in two other directions, one pair to each remaining O. One double bond and two single bonds on the center atom are considered to be 3 electron groups. Remember the negative two charge is distributed over the whole ion. return to top |

|
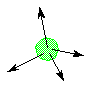
The VSEPR theory table below refers to electron groups around the central atom in a structure There is a descriptive name for each electron group geometry or arrangement of the electron pairs around the center atom. The sketch indicates the electron groups around the central atom only.return to top |
|
number of electron groups -----------------> |
|
|
4 |
|
name of geometry of electron groups |
|
|
|
|
sketch of geometry -- electron groups represented by arrows |
|
|
|
Format in AXnEm
|
AX2E0
|
AX3E0
|
AX4E0
|
|
ideal angles between electron groups |
|
|
|
|
The name for the overall shape of a particle may not be the same as the name for the geometry of its electron groups. This is an important because the shape is dictated by the positions of both unshared electron pairs and electrons attaching atoms to the central atom . If all electron pairs on the center atom are used for , then the overall shape of the molecule or ion has the same name as the electron group geometry around the center atom. If there are lone pairs, a double bond, or a triple bond on the center atom, then the name for the overall particle shape is different from the name of the electron group geometry.. return to top |
|
Geometry of electron groups |
|
Ideal bond angles |
|
|
|
Appearance |
name of molecule shape |
|
|
Linear AX2 or AX2E0 |
|
linear |
180o |
|
*All diatomic or 2-atom molecules are linear regardless of the number of electron groups around the "central" atom. return to top |
|
Geometry of electron groups |
|
Ideal bond angles |
|
|
|
Appearance |
name of molecule shape |
|
|
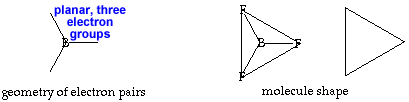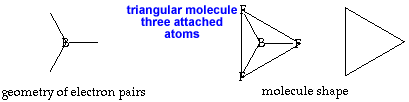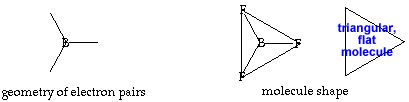
Trigonal planar AX3 or AX3E0 |
|
triangular planar |
120o |
|
AX2E1 |
|
angular |
120o |
|
AX1E2 |
|
linear |
120o |
|
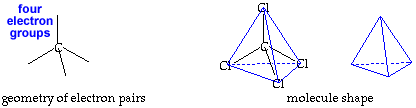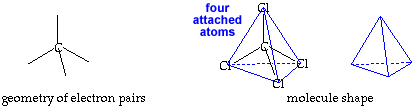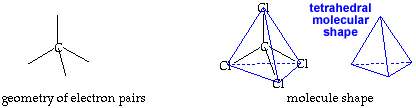
Tetrahedral AX4E0 |
|
tetrahedral |
109.5o |
|
AX3E1 |
|
pyramidal |
109.5o |
|
AX2E2 |
|
angular, bent |
109.5o |
|
AX1E3 |
|
linear |
109.5o |
|
|
|||
|
|
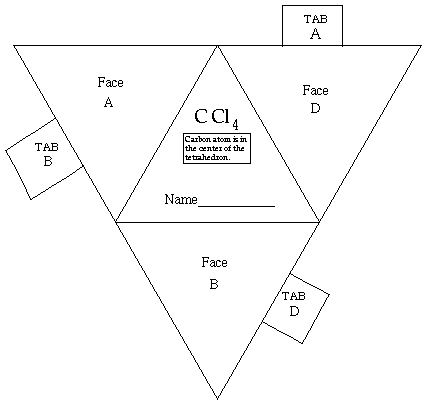
Use a ruler and a ball-point pen to scribe the lines that mark where folds need to be made. You do the scribing by lining up the ruler along the fold line and running the ball point pen tip along the printed lines. This "etches" the paper. Scribing the edges makes it easier to have the right positions for the folds. Cut out each paper model. Rememberdo not cut off the black lines. |
|
Cut out the planar triangle. No folding is needed since this shape is flat. AX3 or AX3E0 Boron trifluoride, BF3, is an example of the planar triangular shape. Boron, unlike most nonmetals, often has only 6 electrons in its valence shell, giving it only 3 pairs instead of 4. return to top |
Tetrahedral Shape AX4E0click for pladoh model
| Be careful to keep the A, B, and D tabs on the template when you cut out the tetrahedron. They will be folded against a corresponding face and taped down to maintain the shape of the model. Be sure to leave the black edges on the faces. Write your name on the line provided. Hold the cutout so you can read your name. Fold faces A, B, and D away from you. Fold tab B over face B and secure tab with transparent tape. Fold tab A over face A and secure with tape. Likewise, fold tab D over face D and secure with tape. You now have a paper model of a tetrahedron. Carbon tetrachloride, CCl4, is a molecule shaped like a tetrahedron. It has a chlorine atom at each of the four points of the tetrahedron. A carbon atom is in the center of the tetrahedron. In your model of the tetrahedron, the C atom would be hidden inside the paper model. The bonds from C to each Cl are also hidden inside. return to top 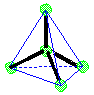
|
|
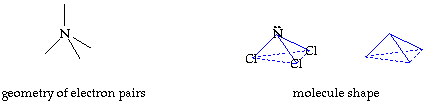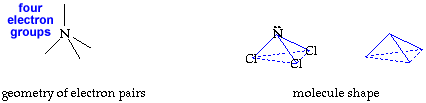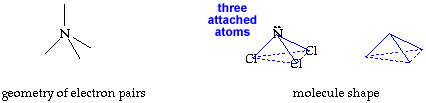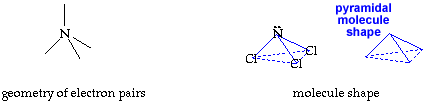
Write your name on the line. Hold the cutout so you can read your name. Fold faces A and B away from you. Hold face C up so you can read it. Fold the tab on face B over face A. Secure the tab and edges with transparent tape. You now have your trigonal pyramid molecule shape. return to top
The molecule, NCl3, has a trigonal pyramid shape. The nitrogen is at the top of the pyramid. The central nitrogen atom has an octet with 3 pairs of electrons used for the three N-Cl bonds and the other two electrons in a lone pair. |
|
|
|
|
|
|
Pladoh™, 2 cans Use 2 different colors of Pladoh™ If you do not want to use Pladoh you can substitute marshmallows, gum drops, styrofoam balls or similar materials. toothpicks return to top |
|
Three dimensional models can be made using toothpicks, marshmallows or spheres made from Playdoh. The spheres represent the atoms in the particle. The toothpicks represent the electron pairs around the central atom. Open a can of Playdoh and remove a piece that is about one inch in diameter. Roll the Playdoh around between the palms of you hands, making a circular motion with your palms. The lump will gradually roll into a sphere. Repeat this process to make a total of 10 Play-doh spheres of this color . Open the other can of Play-doh, take out a lump that is about 1/2 inch in diameter, and roll this into a sphere. Make 16 or 20 of these spheres. The large number of balls (spheres) are needed if all the models are kept during the procedure. |
|
Make a model of the linear geometry of electron groups around a central atom. return to top
|
|
Make a molecule model with a linear shape. Now stick a small sphere onto the open end of each toothpick. You should be able to imagine a straight line from one small sphere, through the large sphere, and on to the other small sphere. Thus the two electron clouds contained in a simple triatomic molecule AX2 will extend out in opposite directions; an angular separation of 180° places the two bonding orbitals as far away from each other they can get. This molecule shape is called "linear". Note there is a"large" sphere in the middle.
|
|
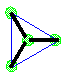
Make a model of the triangular planar geometry of electron groups around a central atom.

|
|
Make a molecule model with a triangular planar shape.
|
|
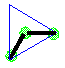
Make a molecule model with an angular or bent shape.
|
|
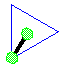
Make a molecule model with a linear shape.
|
|
Make a model of the tetrahedral geometry of electron groups around a central atom. AX4

|
|
Make a molecule model with a tetrahedral shape, AX4.click for AX4 pladoh mode Using your first model of the tetrahedral electron pair geometry, stick a small sphere onto the open end of each toothpick. If you connected the small spheres, X, you would get a tetrahedron. The central atom, A, and the bonds would be inside this tetrahedron. If you drew a line from one small sphere to the central atom and then on to another small sphere, you would have drawn an angle of 109.5o. This molecule shape is called "tetrahedral" . This is also represented by identifying the central atom represented by A and the attached atoms by X the representation AX4. 
|
|
Make a molecule model with a triangular pyramid shape AX3E1 Stick a small sphere onto the open end of 3 toothpicks. If you connected the small spheres, you would get a triangle with 3 equal sides. If you then connected each small sphere to the big sphere, you would get a pyramid with this triangle for its base. The big sphere would be at the top of the pyramid. Notice that the pyramid molecule is not the same as the tetrahedral molecule. This molecule shape is a "triangular pyramid". There is one lone pair pointing to one of the corners of the tetrahedron. 
|
|
Make a molecule model with an angular shape or bent shape. AX2E2 Now stick a small sphere onto the open end of 2 of the toothpicks. If you drew a line from one small sphere to the central atom and then on to the other small sphere, you would get an angle or a bent line; the size of this angle is 109.5o. This molecule shape is called "angular". There are two lone pairs pointing to two of the corners of the tetrahedron. 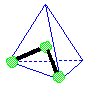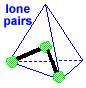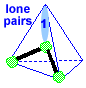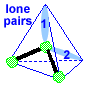
|
|
Make a molecule model with a linear shape. AX1E3
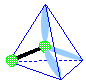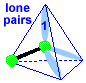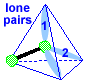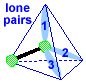
|
|
|
Applying VSEPR to Real Molecules

|
Your observations from the models of these molecules are needed to answer the questions in the report sheet. For each of the molecules listed below:
|
|
|
| Methane, CH4 Ammonia, NH3 Water, H2O Hydrogen Fluoride, HF |
|
|
|
Carbon Dioxide, CO2 |
Ethylene, C2H4 |
|
Formaldehyde, H2CO |
Acetylene, C2H2 |
|
Hydrogen Cyanide, HCN |
nitric oxide, NO |
|
When you are finished with the activities listed your report sheet, stuff the Play-doh™ back into its original containers and reclose it tightly so the Play-doh™ does not dry out. Follow the storage directions on the container. Save the Play-doh™ for other experiments. return to top |
| Complete the following work sheet using your models and sketches. The report is submitted using the equiz in Angel. The work sheet is supposed to help complete the equiz. |
VSEPR Theory Report Sheet return to top
|
----------------------------------------------------------------------------------------------- |
Name______________ |
Molecules with only single bonds
|
|
Methane, CH4 |
Ammonia, NH3 |
Water, H2O |
Hydrogen Fluoride, HF |
|
Number of valence electrons in the molecule |
___________ |
___________ |
___________ |
___________ |
|
Number of valence electrons around central atom in the molecule |
___________ |
___________ |
___________ |
___________ |
|
# of single bonds on central atom |
___________ |
___________ |
___________ |
___________ |
|
# of lone pairs on central atom |
___________ |
___________ |
___________ |
___________ |
|
# of double bonds on central atom |
___________ |
___________ |
___________ |
___________ |
|
# of triple bonds on central atom |
___________ |
___________ |
___________ |
___________ |
|
# of electron groups on central atom |
___________ |
___________ |
___________ |
___________ |
|
Name of geometry of electron pairs |
___________ |
___________ |
___________ |
___________ |
|
Name of molecule shape |
___________ |
___________ |
___________ |
___________ |
|
What molecule shape do you expect for each compound listed below based on the models and examples above? return to top |
|
compound |
shape (bent,
linear, tetrahedral, trigonal pyramidal) |
AXnEm type (AX4E0, AX3E1, AX2E2, AX1E3) |
|
Hydrogen Sulfide, H2S |
___________ |
___________ |
|
Hydrogen Chloride, HCl |
___________ |
___________ |
|
Phosphine, PH3 |
___________ |
___________ |
|
Silane, SiH4 |
___________ |
___________ |
Molecules with both single and multiple bonds
Nitrogen oxide, NO |
Carbon Dioxide, CO2 |
Formaldehyde, H2CO |
Hydrogen Cyanide, HCN |
|
Total # valence electrons in the molecule |
___________ |
___________ |
___________ |
___________ |
AXE classification of central atom |
___________ |
___________ |
___________ |
___________ |
Total valence electrons around central atom in the molecule |
___________ |
___________ |
___________ |
___________ |
# of single bonds on central atom |
___________ |
___________ |
___________ |
___________ |
# of double bonds on central atom |
___________ |
___________ |
___________ |
___________ |
# of triple bonds on central atom |
___________ |
___________ |
___________ |
___________ |
# of electron groups on central atom |
___________ |
___________ |
___________ |
___________ |
Name of geometry of electron pairs |
___________ |
___________ |
___________ |
___________ |
Name of molecule shape linear, tetrahedral, planar, bent, etc |
___________ |
___________ |
___________ |
___________ |
Ethylene and acetylene molecules with two central atoms
|
Name the geometry of the electron pairs(groups) around each of the individual C atoms in Ethylene, C2H4.
Justify your answer. |
____________________ |
Describe the overall shape of the molecule Ethylene, C2H4. Linear, tetrahedral, planar, bent, etc. Justify your answer. |
____________________ |
|
Name the geometry of the electron pairs(groups) around each of the individual C atoms in Acetylene, C2H2. Justify your answer. |
____________________ |
|
Describe the overall shape of the molecule Acetylene, C2H2. Linear, tetrahedral, planar, bent, etc. Justify your answer. |
____________________ |
All rights reserved revised July 9, 2013 return to top
Dr. Walt Volland
vsepr tetrahedral-pladoh-models